Abstract
Background
In order to meet the unmet needs of rare disease patients in China, importing orphan drugs is an important way. The objectives of this study were to investigate the marketing trend of orphan drugs approved by the US Food and Drug Administration (FDA) and imported by China, to examine the orphan drug lag between China and the United States.
Methods
This study analyzes the orphan drugs approved by FDA and imported by China from January 2010 to December 2021. The approval lag for orphan drugs between China and the US was calculated and analyzed by approval time. Factors potentially affecting the approval lag, such as target disease, ATC classification, formulation, corporation name, drug type, and whether the indications belong to the first batch of rare diseases catalogue were investigated.
Results
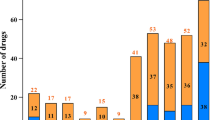
The number of FDA-approved orphan drugs imported by China is increasing year by year, and the approval lag of these drugs is gradually decreasing, especially in the classification of Non-L, Injections, Non-United States, and biological product. Compared with 2010–2015, the approval lag of total drugs in the study was significantly improved in 2016–2021 (1977 days) compared with 2010–2015 (3928 days).
Conclusion
China’s groundbreaking regulatory reforms of drugs since 2015 had made significant progress in reducing orphan drug lags, but there is still considerable room for progress. We should more actively promote the approval of rare disease drugs in China, establish a better approval mechanism, and enable Chinese patients with rare diseases to receive drug treatment in a timely manner.


Similar content being viewed by others
References
Song P, He J, Li F, Jin C. Innovative measures to combat rare diseases in China: the national rare diseases registry system, larger-scale clinical cohort studies, and studies in combination with precision medicine research. Intractable Rare Dis Res. 2017;6:1–5.
Zhou S, Lang J, Wu J, Jin Y. Massive intraperitoneal effusion caused by rare disease: a case report. Asian J Surg. 2022;2022:1015.
Li XQ, Peng XX, Gong CX. Access to orphan drugs is a challenge for sustainable management of cystinosis in China. Chin Med J. 2018;131(19):2388–9.
Mu Y, Song K, Song Y. A cross-sectional study of price and affordability of drugs for rare diseases in Shandong Province, China. Int J Environ Res Public Health. 2022;19(20):13319.
Rawson NSB. Canadian, European and United States new drug approval times now relatively similar. Regul Toxicol Pharmacol. 2018;96:121–6.
Hirai Y, Kinoshita H, Kusama M, Yasuda K, Sugiyama Y, Ono S. Delays in new drug applications in Japan and industrial R&D strategies. Clin Pharmacol Ther. 2010;87(2):212–8.
Shao L, Xu L, Li Q, Chakravarthy R, Yang Z, Kaitin KI. Regulatory watch: innovative drug availability in China. Nat Rev Drug Discov. 2016;15(11):739–40.
Ueyama E, Kaneko M, Narukawa M. Analysis of pediatric drug approval lag in Japan. Ther Innov Regul Sci. 2021;55(2):336–45.
Luo X, Du X, Li Z, Qian F, Yang Y. Assessment of the delay in novel anticancer drugs between China and the United States: a comparative study of drugs approved between 2010 and 2021. Clin Pharmacol Ther. 2022;113:170.
Search Orphan Drug Designations and Approvals, U.S. Food and Drug Administration. 2022. https://www.accessdata.fda.gov/scripts/opdlisting/oopd/index.cfm. Accessed 24 May 2022.
Center for Drug Evaluation, National Medical Products Administration. 2022. https://www.cde.org.cn/main/xxgk/listpage/b40868b5e21c038a6aa8b4319d21b07d. Accessed 24 May 2022.
Drug Database. YAOZH. 2022. https://www.yaozh.com/. Accessed 24 May 2022.
Tripathy S, Murthy PN, Patra BP, Mehra P, Dureja H. Challenges and prospects in Chinese pharmaceutical regulatory environment. J Gener Med. 2021;17(3):106–14.
Bajaj G, Gupta M, Wang HH, et al. Challenges and opportunities with oncology drug development in China. Clin Pharmacol Ther. 2019;105:363–75.
Yamashita K, Kaneko M, Narukawa M. A significant anticancer drug approval lag between Japan and the United States still exists for minor cancers. Clin Pharmacol Ther. 2019;105:153–60.
Zhu X, Liu B. Launch delay of new drugs in China and effect on patients’ health. Clin Ther. 2020;42:1750–61.
China General Office of the State Council. Opinions on deepening the reform of the review and approval system and encouraging the innovation of drugs and medical devices. https://www.nmpa.gov.cn/xxgk/fgwj/gzwj/gzwjyp/20171009164201907.html. Accessed 24 May 2022.
Zhao S, et al. Time to raise the bar: transition rate of phase 1 programs on anticancer drugs. Cancer Cell. 2022;40:233–5.
Yao X, Ding J, Liu Y, Li P. The new drug conditional approval process in China: challenges and opportunities. Clin Ther. 2017;39(5):1040–51.
Li G, Qin Y, Xie C, Wu YL, Chen X. Trends in oncology drug Innovation in China. Nat Rev Drug Discov. 2021;20:15–6.
Shao L, Xu L, Li Q, Chakravarthy R, Yang Z, Kaitin KI. Regulatory watch: innovative drug availability in China. Nat Rev Drug Discov. 2016;15:739–40.
Li G, Liu X, Chen X. Simultaneous development of zanubrutinib in the USA and China. Nat Rev Clin Oncol. 2020;17:589–90.
Zhang S, Chen L, Zhang Z, Zhao Y. Orphan drug development in China: progress and challenges. Lancet (Lond, Engl). 2019;394:1127–8.
Cheng A, Xie Z. Challenges in orphan drug development and regulatory policy in China. Orphanet J Rare Dis. 2017;12:13.
Xu L, Gao H, Kaitin KI, Shao L. Reforming China’s drug regulatory system. Nat Rev Drug Discov. 2018;17:858–9.
Li X, Yang Y. The drug lag issue: a 20-year review of China. Investig New Drugs. 2021;39(5):1389–98.
National Medical Products Administration. Review and approval of new drugs outside of China for urgent clinical needs. 2018. https://www.nmpa.gov.cn/zhuanti/ypqxgg/ggzhcfg/20181.03017.12016.html. Accessed 24 May 2022.
Center for Drug Evaluation, National Medical Products Administration. 2022. https://www.cde.org.cn/main/news/viewInfoCommon/c4e1ef312a0a0c039a7a4ca55b91d4e8. Accessed 1 May 2023.
Hwang TJ, Kesselheim AS, Tibau A, Lee CC, Vokinger KN. Clinical benefit and expedited approval of cancer drugs in the United States, European Union, Switzerland, Japan, Canada, and Australia. JCO Oncol Pract. 2022;18: e1522.
National Medical Products Administration. 2018. https://www.nmpa.gov.cn/xxgk/ggtg/qtggtg/20180523110601517.html. Accessed 1 May 2023.
Nakayama H, Matsumaru N, Tsukamoto K. The drug lag and associated factors for orphan anticancer drugs in Japan compared to the United States. Investig New Drugs. 2019;37(5):1086–93.
Xu L, Gao H, Kaitin KI, Shao L. Reforming China’s d rugregulatory system. Nat Rev Drug Discov. 2018;17:858–9.
Chaput B, Eburdery H, Crouzet C, Grolleau JL, Chavoin JP, Garrido I. Macrolane®: a severe case of calf cellulitis after modeling injection. Ann Chir Plast Esthet. 2012;57(1):83–6.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peng, F., Zheng, H. Analysis on the Marketing Trend and Approval Lag of Imported Orphan Drugs from 2010 to 2021 in China. Ther Innov Regul Sci 57, 1314–1321 (2023). https://doi.org/10.1007/s43441-023-00572-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43441-023-00572-8