Abstract
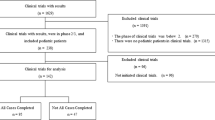
Although the percentage of multi-regional clinical trials (MRCTs) submitted for drug approval in Japan increased significantly since the 2007 publication of the regulatory guideline, “Basic principles on global clinical trials”, strategic collaborations between Asian countries will be important to promote MRCTs in accordance with the ICH E17 guideline published in 2017. In this study, characteristics of MRCTs reviewed for drug approval in Japan, especially those with participation by South-East Asia and East Asia, were investigated to explore opportunities for collaborations on global drug development in Asia. More than 90% of reviewed trials were conducted as global MRCTs. In addition to Japan, South-East Asia has participated in various types of MRCTs in terms of total numbers of subjects and countries. However, South-East Asia participation was lower in large-size MRCTs (total sample size ≥ 1000) than in middle- (500 ≤ total sample size < 1000) and small-size MRCTs (total sample size < 500). Furthermore, similar clinical trials for the same indications to the MRCTs without South-East Asia were rarely conducted separately in South-East Asia. Participation of other Asian countries did not affect the percentage of Japanese subjects enrolled in an MRCT, but did significantly increase the percentage of participating Asian subjects. These results suggest that additional opportunities for collaboration on MRCTs may be possible between Japan and other Asian countries, especially more collaborations with South-East Asia in the large-size MRCTs. More data of Asian populations from MRCTs will be useful for exploring an important ethnic factor affecting drug response, and will provide a sound scientific basis in considering the application of the pooled data concept in Asia, as described in the ICH E17 guideline.



Similar content being viewed by others
Data Availability
Not applicable.
References
Ministry of Health Labour and Welfare. Basic principles on global clinical trials (0928010 Evaluation and Licensing Division, Pharmaceuticals and Food Safety Bureau ). http://www.pmda.go.jp/files/000157900.pdf (2007). Accessed 15 May 2023.
Asano K, Uyama Y, Tohkin M. Factors affecting drug-development strategies in Asian global clinical trials for drug approval in Japan. Clin Transl Sci. 2018;11:182–8.
Miyazaki K, Sato Y, Hanaoka H, Uyama Y. Current Status and Open Issues Concerning Global Clinical Trials (GCTs) in Japan and East Asia. Clin Transl Sci. 2017;10:503–8.
Hasunuma T, et al. Absence of ethnic differences in the pharmacokinetics of moxifloxacin, simvastatin, and meloxicam among three East Asian populations and Caucasians. Br J Clin Pharmacol. 2016;81:1078–90.
Yasuda SU, Zhang L, Huang SM. The role of ethnicity in variability in response to drugs: focus on clinical pharmacology studies. Clin Pharmacol Ther. 2008;84:417–23.
Kurose K, Sugiyama E, Saito Y. Population differences in major functional polymorphisms of pharmacokinetics/pharmacodynamics-related genes in eastern Asians and Europeans: implications in the clinical trials for novel drug development. Drug Metab Pharmacokinet. 2012;27:9–54.
Pharmaceuticals and Medical Devices Agency. List of Approved Products. https://www.pmda.go.jp/english/review-services/reviews/approved-information/drugs/0002.html. Accessed 15 May 2023.
Pharmaceuticals and Medical Devices Agency, Information search for new drugs, such as review reports, package inserts, and other related infromation. [in Japanese]. https://www.pmda.go.jp/PmdaSearch/iyakuSearch/ (2022). Accessed 15 May 2023.
Clinical Trials.gov. https://www.clinicaltrials.gov/. Accessed 15 May 2023.
Ministry of Health Labour and Welfare, Basic Principles on Global Clinical Trials (Reference Cases) (Administrative Notice, Evaluation and Licensing Division, Pharmaceuticals and Food Safety Bureau). https://www.pmda.go.jp/files/000152969.pdf. (2012). Accessed 15 May 2023.
ICH E17 guideline, ICH official web-site. https://ich.org/page/efficacy-guidelines#16. Accessed 15 May 2023.
Sai K, et al. Population/regional differences in efficacy of three drug categories (antidiabetic, respiratory, and psychotropic agents) among East Asians: a retrospective study based on multi-regional clinical trials. Br J Clin Pharmacol. 2019;85:1270–82.
Zhou X, et al. Asia-inclusive global development of pevonedistat: Clinical pharmacology and translational research enabling a phase 3 multiregional clinical trial. Clin Transl Sci. 2021;14:1069–81.
Venkatakrishnan, K. et al. Asia-Inclusive clinical research and development enabled by translational science and quantitative clinical pharmacology: toward a culture that challenges the status quo. Clin Pharmacol Ther, (2022).
Jeon I, et al. The necessary conduct: exploratory multiregional clinical trials in East Asia. Clin Transl Sci. 2021;14:2399–407.
US Food and Drug Administration, Draft guidance for industry, Diversity Plans to Improve Enrollment of Participants from Underrepresented Racial and Ethnic Populations in Clinical Trials https://www.fda.gov/media/157635/download. (2022). Accessed 15 May 2023.
Asano K, Aoi Y, Kamada S, Uyama Y, Tohkin M. Points to consider for implementation of the ICH E17 guideline: learning from past multi-regional clinical trials (MRCTs) in Japan. Clin Pharmacol Ther. 2021;109:1555–63.
Sonoda M, Urbiztondo MRU, Siburian MD, Kerdsakundee N, Muchanga SMJ, Iiyama T. Boosting multiregional clinical trials (MRCT) in Asia through the establishment of the Japan-led network for clinical research, the ARO alliance for ASEAN & East Asia (ARISE). Glob Health Med. 2022;4:247–9.
Noguchi A, Hanaoka H, Uyama Y. Potential future drug development Lag in Japan based on an analysis of multiregional clinical trials in the US, Europe, and East Asia. Ther Innov Reg Sci. 2022;56:523–9.
Su L, Liu S, Li G, Xie C, Yang H, Liu Y, Yin C, Chen X. Trends and characteristics of new drug approvals in China, 2011–2021. Ther Innov Regul Sci. 2023;57(2):343–51.
Lee SW, et al. Notable differences in drug lag between Korea and Japan of new drugs between 2009 and 2017. Ther Innov Regul Sci. 2020;54:418–23.
Acknowledgements
The views expressed herein are the result of independent work and do not necessarily represent the views and findings of the Pharmaceuticals & Medical Devices Agency.
Funding
This research was partially supported by the research grant on regulatory science of pharmaceuticals and medical devices from the Ministry of Health, Labour and Welfare (20KC2010).
Author information
Authors and Affiliations
Contributions
YA and YU designed the studies, and YA, YK, KA and YO performed and analyzed the studies, and YA and YU wrote manuscript. All authors read, approved, and contributed to the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aoi, Y., Kato, Y., Asano, K. et al. Characteristics of Asian Participation in Multi-regional Clinical Trials Reviewed for Drug Approval in Japan: Opportunities for Collaboration Between South-East Asia, East Asia, and Japan. Ther Innov Regul Sci 57, 1298–1303 (2023). https://doi.org/10.1007/s43441-023-00566-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43441-023-00566-6