Abstract
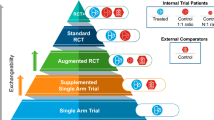
The use of information from real world to assess the effectiveness of medical products is becoming increasingly popular and more acceptable by regulatory agencies. According to a strategic real-world evidence framework published by U.S. Food and Drug Administration, a hybrid randomized controlled trial that augments internal control arm with real-world data is a pragmatic approach worth more attention. In this paper, we aim to improve on existing matching designs for such a hybrid randomized controlled trial. In particular, we propose to match the entire concurrent randomized clinical trial (RCT) such that (1) the matched external control subjects used to augment the internal control arm are as comparable as possible to the RCT population, (2) every active treatment arm in an RCT with multiple treatments is compared with the same control group, and (3) matching can be conducted and the matched set locked before treatment unblinding to better maintain the data integrity and increase the credibility of the analysis. Besides a weighted estimator, we also introduce a bootstrap method to obtain its variance estimation. The finite sample performance of the proposed method is evaluated by simulations based on data from a real clinical trial.
Similar content being viewed by others
Data Availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
FDA. Real-World Evidence. www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence; 2021.
FDA. Complex Innovative Trial Design Pilot Meeting Program. www.fda.gov/drugs/development-resources/complex-innovative-trial-design-pilot-meeting-program; 2021.
FDA. Framework for FDA’s real-world evidence program. https://www.fda.gov/media/120060/download; 2018.
Pocock SJ. The combination of randomized and historical controls in clinical trials. J Chronic Dis. 1976;29(3):175–88. https://doi.org/10.1016/0021-9681(76)90044-8.
Chen MH, Ibrahim JG. Power prior distributions for regression models. Stat Sci. 2000;15(1):46–60. https://doi.org/10.1214/ss/1009212673.
Duan Y, Ye K, Smith EP. Evaluating water quality using power priors to incorporate historical information. Environmetrics. 2006;17(1):95–106. https://doi.org/10.1002/env.752.
Neuenschwander B, Branson M, Spiegelhalter DJ. A note on the power prior. Stat Med. 2009;28(28):3562–6. https://doi.org/10.1002/sim.3722.
Banbeta A, Rosmalen VJ, Dejardin D, Lesaffre E. Modified power prior with multiple historical trials for binary endpoints. Stat Med. 2019;38(7):1147–69. https://doi.org/10.1002/sim.8019.
Lin J, Gamalo-Siebers M, Tiwari R. Propensity-score-based priors for Bayesian augmented control design. Pharm Stat. 2019;18(2):223–38. https://doi.org/10.1002/pst.1918.
Wang C, Li H, Chen WC, et al. Propensity score-integrated power prior approach for incorporating real-world evidence in single-arm clinical studies. J Biopharm Stat. 2019;29(5):731–48. https://doi.org/10.1080/10543406.2019.1657133.
Neuenschwander B, Capkun-Niggli G, Branson M, Spiegelhalter DJ. Summarizing historical information on controls in clinical trials. Clin Trials. 2010;7(1):5–18. https://doi.org/10.1177/1740774509356002.
Schmidli H, Gsteiger S, Roychoudhury S, O’Hagan A, Spiegelhalter D, Neuenschwander B. Robust meta-analytic-predictive priors in clinical trials with historical control information. Biometrics. 2014;70(4):1023–32. https://doi.org/10.1111/biom.12242.
Hupf B, Bunn V, Lin J, Dong C. Bayesian semiparametric meta-analytic-predictive prior for historical control borrowing in clinical trials. Stat Med. 2021;40(14):3385–99. https://doi.org/10.1002/sim.8970.
Liu M, Bunn V, Hupf B, Lin J, Lin J. Propensity-score-based meta-analytic predictive prior for incorporating real-world and historical data. Stat Med. 2021;40(22):4794–808. https://doi.org/10.1002/sim.9095.
Rosmalen VJ, Dejardin D, Norden VY, Löwenberg B, Lesaffre E. Including historical data in the analysis of clinical trials: is it worth the effort? Stat Methods Med Res. 2018;27(10):3167–82. https://doi.org/10.1177/0962280217694506.
Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. https://doi.org/10.1093/biomet/70.1.41.
Robins JM, Rotnitzky A, Zhao LP. Estimation of regression coefficients when some regressors are not always observed. J Am Stat Assoc. 1994;89(427):846–66.
Hirano K, Imbens GW. Estimation of causal effects using propensity score weighting: an application to data on right heart catheterization. Health Serv Outcomes Res Methodol. 2001;2(3):259–78.
Hainmueller J. Entropy balancing for causal effects: a multivariate reweighting method to produce balanced samples in observational studies. Polit Anal. 2012;20(1):25–46.
Zhao Q, Percival D. Entropy balancing is doubly robust. J Causal Inference. 2017;5(1):20160010. https://doi.org/10.1515/jci-2016-0010.
Imai K, Ratkovic M. Covariate balancing propensity score. J R Stat Soc Ser B. 2014;76(1):243–63.
Abadie A, Imbens GW. Large sample properties of matching estimators for average treatment effects. Econometrica. 2006;74(1):235–67.
Abadie A, Imbens GW. Matching on the estimated propensity score. Econometrica. 2016;84(2):781–807. https://doi.org/10.3982/ECTA11293.
Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(1):33–8.
Gu XS, Rosenbaum PR. Comparison of multivariate matching methods: structures, distances, and algorithms. J Comput Graphical Stat. 1993;2(4):405–20.
Rosenbaum PR. Design of Observational Studies. New York: Springer; 2020.
Stuart EA, Rubin DB. Matching With multiple control groups with adjustment for group differences. J Educ Behav Stat. 2008;33(3):279–306. https://doi.org/10.3102/1076998607306078.
Yuan J, Liu J, Zhu R, Lu Y, Palm U. Design of randomized controlled confirmatory trials using historical control data to augment sample size for concurrent controls. J Biopharm Stat. 2019;29(3):558–73. https://doi.org/10.1080/10543406.2018.1559853.
Schafer JL, Kang J. Average causal effects from nonrandomized studies: a practical guide and simulated example. Psychol Methods. 2008;13(4):279–313. https://doi.org/10.1037/a0014268.
Austin PC, Small DS. The use of bootstrapping when using propensity-score matching without replacement: a simulation study. Stat Med. 2014;33(24):4306–19. https://doi.org/10.1002/sim.6276.
Imbens GW. Nonparametric estimation of average treatment effects under exogeneity: a review. Rev Econ Stat. 2004;86(1):4–29. https://doi.org/10.1162/003465304323023651.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Du, Y., Liu, H. et al. An Improved Matching Practice for Augmenting a Randomized Clinical Trial with External Control. Ther Innov Regul Sci 57, 611–618 (2023). https://doi.org/10.1007/s43441-023-00497-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43441-023-00497-2