Abstract
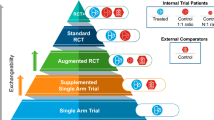
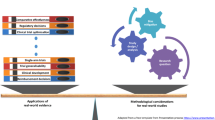
External comparators, also referred to as historical or synthetic controls, present transformational opportunities for broad context and insights alongside clinical research results. The recent confluence of access to quality real-world data (RWD), advanced epidemiologic methods, and legislative directives to regulators for expanded use of RWD is increasing interest in real-world external comparators, opening the door to achieve broader generalizability and learn more, faster. In this less standardized area of research, tailored scientific methodology must be applied for external comparators to accomplish clinical development objectives. Here, we describe methodological considerations for design and illustrate how RWD comparators have been used for regulatory and reimbursement decisions.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mack, C., Christian, J., Brinkley, E. et al. When Context Is Hard to Come By: External Comparators and How to Use Them. Ther Innov Regul Sci 54, 932–938 (2020). https://doi.org/10.1007/s43441-019-00108-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43441-019-00108-z