Abstract
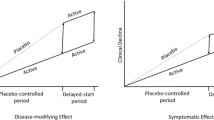
Alzheimer’s disease (AD) has increasingly been recognized as a huge unmet medical need. Currently, there is no approved drug to cure, prevent, or even slow down the disease. It is imperative to develop disease-modifying treatments for AD to alter the underlying disease progression. This paper reviews the most up-to-date regulatory guidance on how to demonstrate disease modification and provides an overview of available methodologies and applications to clinical trials. The intent is to assist the field with future clinical trials designed to demonstrate disease-modifying effect in AD. The methodologies may be generalizable to broader neurodegenerative diseases.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu-Seifert, H., Schumi, J., Miao, X. et al. Disease Modification in Alzheimer’s Disease: Current Thinking. Ther Innov Regul Sci 54, 396–403 (2020). https://doi.org/10.1007/s43441-019-00068-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43441-019-00068-4