Abstract
Background:
Confirmatory phase III trials aim to provide decisive evidence about a medical product’s safety and efficacy. Although these trials are planned and conducted based on accumulated knowledge, they are not without risk or uncertainty. A trial prematurely concluding contributes to great loss in both financial and human research resources.
Methods:
We categorized and evaluated trials concluded prematurely after recruitment had begun, as registered in Clinical Trials.gov between January 2013 and August 2017.
Results:
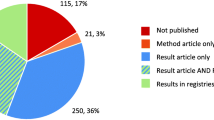
We found 9828 registered interventional phase III trials; of those, 320 were concluded prematurely. Many clinical trials were concluded prematurely for reasons related to reducing participant risk, such as interim stopping for safety, efficacy, or futility. Yet, 70% trials were halted for other reasons, such as insufficient recruitment (the most often cited reason) or unspecified business decisions. Of all prematurely concluded trials, 102 trials evaluated 72 different novel therapeutics; in 66.7% of these trials, the clinical development program was stopped entirely. Most of the prematurely concluded trials (78%) had not provided results to ClinicalTrials.gov at the time of this analysis.
Conclusions:
Evaluation of the factors that influence premature conclusion could inform solutions for improving research participation and help ensure trial completion. Registering and reporting results acknowledges the voluntary contribution and consent expectations of research participants.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guinn, D., Wilhelm, E.E. & Shoulson, I. Reasons for Premature Conclusion of Late Phase Clinical Trials: An Analysis of ClinicalTrials.gov Registered Phase III Trials. Ther Innov Regul Sci 54, 232–239 (2020). https://doi.org/10.1007/s43441-019-00050-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43441-019-00050-0