Abstract
Background
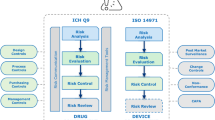
Newly emerging products which combine drugs, devices, and biologics are expected to provide new opportunities in bridging device and drug capabilities and establish synergies while bringing sophisticated combination products to consumers. The emergence of these novel products has triggered new regulatory, strategic, and technological challenges. While progress has been made at clarifying the issues that arise most frequently, regulatory authorities and product developers continue to struggle with complex regulatory and technical issues encompassing the development programs for combination products. A risk-based approach requires not only a strategy but also tools to define key indicators to measure specific risks. Key risk indicators (KRIs) and risk-based quality management systems should focus on safety of research subjects and data integrity.
Methods
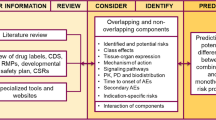
We analyzed current regulatory guidelines throughout the life cycle of combination products and compared old and new approaches to risk-based quality and compliance management for current good manufacturing practices and during pre-clinical and clinical phases of combination products development. Cause–effect analysis for two major risk categories in clinical trials with combination products was performed.
Results
The results of our analysis are based on observations from 15 clinical trials, which were conducted with combination products. Based on our findings, we proposed practical recommendations for the development of KRIs to improve conduct and ensure safety of research subjects in trials with combination products by utilizing risk-based quality management approach.
Conclusion
Combination products, due to their specific nature, can increase risks while being tested in clinical trials. Metrics critical to risks and quality management should be linked to particular processes within development program for combination products. Ongoing collaboration between regulators, industry, and other stakeholders is essential to streamlining of the global combination entities development and approval process in a way that will produce safe and effective products for consumers.




Similar content being viewed by others
References
FDA. Combination product definition. https://www.fda.gov/combination-products/about-combination-products/combination-product-definition-combination-product-types Accessed 14 June 2019.
Lauritsen KJ, Nguyen T. Combination products regulation at the FDA. Clin Pharmacol Ther. 2009;85(5):468–70.
Final rule on combination products cGMPs. Federal Registry/Vol. 78/No. 14. 2013. https://www.govinfo.gov/content/pkg/FR-2013-01-22/pdf/2013-01068.pdf.
CFR Title 21, Part 4. Current good manufacturing practice requirements for combination products. 22 Jan 2013. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?cfrpart=4.
FDA. Early development considerations for innovative combination products. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/early-development-considerations-innovative-combination-products Accessed 14 June 2019.
FDA. Draft guidance on current good manufacturing practice for combination products. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/combination-products-guidance-documents Accessed 14 June 2019.
21 CFR 3.2 (m) Subpart A: assignment of agency component for review of premarket applications.
21 CFR 3.7 (c) Subpart A: assignment of agency component for review of premarket applications. § 3.7. Request for designation.
ICH Guidance for Industry Guidance for Industry Q8(R2) Pharmaceutical Development. https://www.fda.gov/drugs/guidances-drugs/international-council-harmonisation-quality Accessed 19 June 2019.
ICH Guidance for Industry Q9 Quality Risk Management. https://www.fda.gov/drugs/guidances-drugs/international-council-harmonisation-quality. Accessed 19 June 2019.
ICH Guidance for Industry Q10 Pharmaceutical Quality System. https://www.fda.gov/drugs/guidances-drugs/international-council-harmonisation-quality. Accessed 19 June 2019.
Malikova MA. Risk-based quality and compliance management in clinical trials with combination products. Invited presentation at 27th annual European meeting of Drug Information Association, April 13–15, 2015, Paris, France.
E6(R2) Good Clinical Practice: Integrated Addendum to ICH E6(R1) Guidance for Industry. https://www.regulations.gov/document?D=FDA-2018-D-0719-0002.
Gray CF, Larson EW. Chapter 7. Managing risks. In: Larson EW, Gray CF, editors. Project management: managerial process. 5th ed. New York: McGraw Hill; 2011. p. 211–29.
Wills D. The planning paradigm: the marriage between clinical operations and project management. Drug Inf J. 2001;35:1039–42.
Six Sigma: the cause and effect (a.k.a. Fishbone) diagram www.isixsigma.com.
George ML. The lean sigma pocket toolbook: quick reference guide to 100 tools for improving quality and speed. New York: McGraw Hill; 2004.
Murray SL, Grantham K, Damle SB. Development of a generic risk matrix to manage project risks. J Ind Syst Eng. 2011;5(1):35–51.
Disclaimer
Partially, this paper was presented by Malikova M.A. as an invited speaker at 27th Annual European Meeting of Drug Information Association in April of 2015 in Paris, France. The title of presentation was “Risk-Based Quality and Compliance Management in Clinical Trials with Combination Products.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaliaev, A.O., Malikova, M.A. Risk-Based Quality and Compliance Management in Clinical Trials with Combination Products in Changing Global Regulatory Environment. Ther Innov Regul Sci 54, 803–813 (2020). https://doi.org/10.1007/s43441-019-00012-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43441-019-00012-6