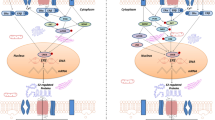
Abstract
Background
Neuropeptide S (NPS) is a multifunctional regulatory factor that exhibits a potent anxiolytic activity in animal models. However, there are no reports dealing with the potential molecular relationships between the anxiolytic activity of selective serotonin reuptake inhibitors (SSRIs) and NPS signaling, especially in the context of novel stress-related neuropeptides action. The present work therefore focused on gene expression of novel stress neuropeptides in the rat brain after acute treatment with escitalopram and in combination with neuropeptide S receptor (NPSR) blockade.
Methods
Studies were carried out on adult, male Sprague–Dawley rats that were divided into five groups: animals injected with saline (control) and experimental rats treated with escitalopram (at single dose 10 mg/kg daily), escitalopram and SHA-68, a selective NPSR antagonist (at a single dose of 40 mg/kg), SHA-68 alone and corresponding vehicle (solvent SHA-68) control. To measure anxiety-like behavior and locomotor activity the open field test was performed. All individuals were killed under anaesthesia and the whole brain was excised. Total mRNA was isolated from homogenized samples of the amygdala, hippocampus, hypothalamus, thalamus, cerebellum, and brainstem. Real-time PCR was used for estimation of related NPS, NPSR, neuromedin U (NMU), NMU receptor 2 (NMUR2) and nesfatin-1 precursor nucleobindin-2 (NUCB2) gene expression.
Results
Acute escitalopram administration affects the local expression of the examined neuropeptides mRNA in a varied manner depending on brain location. An increase in NPSR and NUCB2 mRNA expression in the hypothalamus and brainstem was abolished by SHA-68 coadministration, while NMU mRNA expression was upregulated after NPSR blockade in the hippocampus and cerebellum.
Conclusions
The pharmacological effects of escitalopram may be connected with local NPSR-related alterations in NPS/NMU/NMUR2 and nesfatin-1 gene expression at the level of selected rat brain regions. A novel alternative mode of SSRI action can be therefore cautiously proposed.







Similar content being viewed by others
Data availability statement
The datasets generated during and/or analysed during the following study: Aneta Piwowarczyk-Nowak, Artur Pałasz, Aleksandra Suszka-Świtek, Alessandra Della Vecchia, Aniela Grajoszek, Marek Krzystanek, John J. Worthington. Escitalopram alters local expression of noncanonical stress-related neuropeptides in the rat brain via NPS receptor signaling are available from the corresponding author on reasonable request.
Abbreviations
- ACTH:
-
Adrenocorticotropic hormone
- ARC:
-
Arcuate nucleus
- B2M:
-
Beta-2-microglobulin
- CNS:
-
Central nervous system
- CRH/CRF:
-
Corticotrophin releasing hormone/ factor
- DMH:
-
Dorsomedial hypothalamic nucleus
- DMSO:
-
Dimethyl sulfoxide
- Esc:
-
Escitalopram
- EW:
-
Edinger–Westphal nucleus
- ip:
-
Intraperitoneal
- LC:
-
Locus coeruleus
- NAc:
-
Nucleus accumbens
- NMU:
-
Neuromedin U
- NMUR2:
-
Neuromedin U receptor 2
- NPS:
-
Neuropeptide S
- NPSR:
-
Neuropeptide S receptor
- NUCB2:
-
Nucleobindin 2
- OFT:
-
Open field test
- POMC:
-
Proopiomelanocortin
- PVN:
-
Paraventricular nucleus
- SCN:
-
Suprachiasmatic nucleus
- SHA-68:
-
N-[(4-Fluorophenyl)methyl]tetrahydro-3-oxo-1,1-diphenyl-3H-oxazolo[3,4-a]pyrazine-7(1H)-carboxamide, 3-oxo-1,1-diphenyl-tetrahydro-oxazolo[3,4-a]pyrazine-7-carboxylic acid 4-fluoro-benzylamide, neuropeptide S receptor antagonist
- SON:
-
Supraoptic nucleus
- SSRI:
-
Selective serotonin reuptake inhibitor
- TRH:
-
Thyreotropin-releasing hormone
- VMH:
-
Ventromedial hypothalamus
References
Gupta PR, Prabhavalkar K. Combination therapy with neuropeptides for the treatment of anxiety disorder. Neuropeptides. 2021;86: 102127.
Hökfelt T, Barde S, Xu ZD, Kuteeva E, Rüegg J, Le Maitre E, et al. Neuropeptide and small transmitter coexistence: fundamental studies and relevance to mental illness. Front Neural Circuits. 2018;12:106.
Alldredge B. Pathogenic involvement of neuropeptides in anxiety and depression. Neuropeptides. 2010;44(3):215–24.
Rotzinger S, Lovejoy DA, Tan LA. Behavioral effects of neuropeptides in rodent models of depression and anxiety. Peptides. 2010;31(4):736–56.
Reinscheid RK, Ruzza C. Pharmacology, physiology and genetics of the neuropeptide S system. Pharmaceuticals (Basel). 2021;14(5):401.
Pape HC, Jüngling K, Seidenbecher T, Lesting J, Reinscheid RK. Neuropeptide S: a transmitter system in the brain regulating fear and anxiety. Neuropharmacology. 2010;58(1):29–34.
Reinscheid RK, Xu YL. Neuropeptide S and its receptor: a newly deorphanized G protein-coupled receptor system. Neuroscientist. 2005;11(6):532–8.
Pulkkinen V, Haataja R, Hannelius U, Helve O, Pitkänen OM, Karikoski R, et al. G protein-coupled receptor for asthma susceptibility associates with respiratory distress syndrome. Ann Med. 2006;38:357–66.
Xu YL, Gall CM, Jackson VR, Civelli O, Reinscheid RK. Distribution of neuropeptide S receptor mRNA and neurochemical characteristics of neuropeptide S-expressing neurons in the rat brain. J Comp Neurol. 2007;500(1):84–102.
Clark SD, Duangdao DM, Schulz S, Zhang L, Liu X, Xu YL, Reinscheid RK. Anatomical characterization of the neuropeptide S system in the mouse brain by in situ hybridization and immunohistochemistry. J Comp Neurol. 2011;519(10):1867–93.
Xu YL, Reinscheid RK, Huitron-Resendiz S, Clark SD, Wang Z, et al. Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron. 2004;43(4):487–97.
Tobinski AM, Rappeneau V. Role of the neuropeptide S system in emotionality, stress responsiveness and addiction-like behaviours in rodents: relevance to stress-related disorders. Pharmaceuticals (Basel). 2021;14(8):780.
Beck B, Fernette B, Stricker-Krongrad A. Peptide S is a novel potent inhibitor of voluntary and fast-induced food intake in rats. Biochem Biophys Res Commun. 2005;332(3):859–65.
Cannella N, Economidou D, Kallupi M, Stopponi S, Heiling M, Massi M, et al. Persistent increase of alcohol-seeking evoked by neuropeptide S: an effect mediated by the hypothalamic hypocretin system. Neuropsychopharmacol. 2009;34:2125–34.
Bengoetxea X, Goedecke L, Remmes J, Blaesse P, Grosch T, Lesting J, et al. Human-specific neuropeptide S receptor variants regulate fear extinction in the basal amygdala of male and female mice depending on threat salience. Biol Psychiatry. 2021;90(3):145–55.
Grund T, Neumann ID. Neuropeptide S induces acute anxiolysis by phospholipase C-dependent signaling within the medial amygdala. Neuropsychopharmacology. 2018;43(5):1156–63.
Jüngling K, Seidenbecher T, Sosulina L, Lesting J, Sangha S, Clark SD. Neuropeptide S-mediated control of fear expression and extinction: role of intercalated GABAergic neurons in the amygdala. Neuron. 2008;59(2):298–310.
Leonard SK, Dwyer JM, Sukoff Rizzo SJ, Platt B, Logue SF, Neal SJ, et al. Pharmacology of neuropeptide S in mice: therapeutic relevance to anxiety disorders. Psychopharmacology. 2008;197(4):601–11.
Si W, Aluisio L, Okamura N, Clark SD, Fraser I, Sutton SW, et al. Neuropeptide S stimulates dopaminergic neurotransmission in the medial prefrontal cortex. J Neurochem. 2010;115(2):475–82.
Donner J, Haapakoski R, Ezer S, Melén E, Pirkola S, Gratacòs M, et al. Assessment of the neuropeptide S system in anxiety disorders. Biol Psychiatry. 2010;68(5):474–83.
Domschke K, Reif A, Weber H, Richter J, Hohoff C, Ohrmann P, et al. Neuropeptide S receptor gene—converging evidence for a role in panic disorder. Mol Psychiatry. 2011;16(9):938–48.
Ebner K, Rjabokon A, Pape HC, Singewald N. Increased in vivo release of neuropeptide S in the amygdala of freely moving rats after local depolarisation and emotional stress. Amino Acids. 2011;41(4):991–6.
Fendt M, Imobersteg S, Bürki H, McAllister KH, Sailer AW. Intra-amygdala injections of neuropeptide S block fear-potentiated startle. Neurosci Lett. 2010;474(3):154–7.
Adori C, Barde S, Bogdanovic N, Uhlén M, Reinscheid RR, Kovacs GG, et al. Neuropeptide S- and Neuropeptide S receptor-expressing neuron populations in the human pons. Front Neuroanat. 2015;9:126.
Teranishi H, Hanada R, Neuromedin U. A key molecule in metabolic disorders. Int J Mol Sci. 2021;22(8):4238.
Martinez VG, O’Driscoll L. Neuromedin U: a multifunctional neuropeptide with pleiotropic roles. Clin Chem. 2015;61(3):471–82.
Malendowicz LK, Rucinski M. Neuromedins NMU and NMS: an updated overview of their functions. Front Endocrinol (Lausanne). 2021;12: 713961.
Brighton PJ, Wise A, Dass NB, Willars GB. Paradoxical behavior of neuromedin U in isolated smooth muscle cells and intact tissue. J Pharmacol Exp Ther. 2008;325(1):154–64.
Fujii R, Hosoya M, Fukusumi S, Kawamata Y, Habata Y, Hinuma S, et al. Identification of neuromedin U as the cognate ligand of the orphan G protein-coupled receptor FM-3. J Biol Chem. 2000;275(28):21068–74.
Ballesta J, Carlei F, Bishop AE, Steel JH, Gibson SJ, Fahey M, et al. Occurrence and developmental pattern of neuromedin U-immunoreactive nerves in the gastrointestinal tract and brain of the rat. Neuroscience. 1988;25(3):797–816.
Szekeres PG, Muir AI, Spinage LD, Miller JE, Butler SI, Smith A, et al. Neuromedin U is a potent agonist at the orphan G protein-coupled receptor FM3. J Biol Chem. 2000;275(27):20247–2050.
Howard AD, Wang R, Pong SS, Mellin TN, Strack A, Guan XM, et al. Identification of receptors for neuromedin U and its role in feeding. Nature. 2000;406(6791):70–4.
Hsu SH, Luo CW. Molecular dissection of G protein preference using Gsalpha chimeras reveals novel ligand signaling of GPCRs. Am J Physiol Endocrinol Metab. 2007;293(4):E1021–9.
Shan L, Qiao X, Crona JH, Behan J, Wang S, Laz T, et al. Identification of a novel neuromedin U receptor subtype expressed in the central nervous system. J Biol Chem. 2000;275:39482–6.
Graham ES, Turnbull Y, Fotheringham P, Nilaweera K, Mercer JG, Morgan PJ, et al. Neuromedin U and Neuromedin U receptor-2 expression in the mouse and rat hypothalamus: effects of nutritional status. J Neurochem. 2003;87(5):1165–73.
Gartlon J, Szekeres P, Pullen M, Sarau HM, Aiyar N, Shabon U, et al. Localisation of NMU1R and NMU2R in human and rat central nervous system and effects of neuromedin-U following central administration in rats. Psychopharmacology. 2004;177:1–14.
Hanada R, Nakazato M, Murakami N, Sakihara S, Yoshimatsu H, Toshinai K, et al. A role for neuromedin U in stress response. Biochem Biophys Res Commun. 2001;289(1):225–8.
Tanaka M, Telegdy G. Neurotransmissions of antidepressant-like effects of neuromedin U-23 in mice. Behav Brain Res. 2014;259:196–9.
Telegdy G, Adamik A. Anxiolytic action of neuromedin-U and neurotransmitters involved in mice. Regul Pept. 2013;186:137–40.
Wren AM, Small CJ, Abbott CR, Jethwa PH, Kennedy AR, Murphy KG, et al. Hypothalamic actions of neuromedin U. Endocrinology. 2002;143(11):4227–34.
Zeng H, Gragerov A, Hohmann JG, Pavlova MN, Schimpf BA, Xu H, et al. Neuromedin U receptor 2-deficient mice display differential responses in sensory perception, stress, and feeding. Mol Cell Biol. 2006;26(24):9352–63.
Vallöf D, Kalafateli AL, Jerlhag E. Brain region-specific neuromedin U signalling regulates alcohol-related behav-iours and food intake in rodents. Addict Biol. 2020;25: e12764.
Kasper JM, Smith AE, Hommel JD. Cocaine-evoked locomotor activity negatively correlates with the expression of neuromedin U receptor 2 in the nucleus accumbens. Front Behav Neurosci. 2018;12:271.
Schalla MA, Stengel A. Current understanding of the role of nesfatin-1. J Endocr Soc. 2018;2(10):1188–206.
Pałasz A, Krzystanek M, Worthington J, Czajkowska B, Kostro K, Wiaderkiewicz R, et al. Nesfatin-1, a unique regulatory neuropeptide of the brain. Neuropeptides. 2012;46(3):105–12.
Garcia-Galiano D, Navarro VM, Roa J, Ruiz-Pino F, Sanchez-Garrido MA, Pineda R, et al. The anorexigenic neuropeptide, nesfatin-1, is indispensable for normal puberty onset in the female rat. J Neurosci. 2010;30:7783–92.
Gonzalez R, Kerbel B, Chun A, Unniappan S. Molecular, cellular and physiological evidences for the anorexigenic actions of nesfatin-1 in goldfish. PLoS ONE. 2010;5(12): e15201.
Rupp SK, Wölk E, Stengel A. Nesfatin-1 receptor: distribution, signaling and increasing evidence for a G protein-coupled receptor—a systematic review. Front Endocrinol (Lausanne). 2021;12: 740174.
Stengel A, Goebel M, Tache Y. Nesfatin-1: a novel inhibitory regulator of food intake and body weight. Obes Rev. 2011;12:261–71.
Shimizu H, Oh-I S, Okada S, Mori M. Nesfatin-1: an overview and future clinical application. Endocr J. 2009;56:537–43.
Foo KS, Brismar H, Broberger C. Distribution and neuropeptide coexistence of nucleobindin-2 mRNA/nesfatin-like immunoreactivity in the rat CNS. Neuroscience. 2008;156:563–79.
Pałasz A, Rojczyk E, Siwiec A, Janas-Kozik M. Nesfatin-1 in the neurochemistry of eating disorders. Psychiatr Pol. 2020;54(2):209–22.
Stengel A, Tache Y. Nesfatin-1: role as a possible new potent regulator of food intake. Regul Pept. 2010;9:18–23.
Weibert E, Hofmann T, Stengel A. Role of nesfatin-1 in anxiety, depression and the response to stress. Psychoneuroendocrinology. 2019;100:58–66.
Ge JF, Xu YY, Qin G, Pan XY, Cheng JQ, Chen FH. Nesfatin-1, a potent anorexic agent, decreases exploration and induces anxiety-like behavior in rats without altering learning or memory. Brain Res. 2015;1629:171–81.
Merali Z, Cayer C, Kent P, Anisman H. Nesfatin-1 increases anxiety- and fear-related behaviors in the rat. Psychopharmacology. 2008;201:115–23.
Goebel M, Stengel A, Wang L, Lambrecht NW, Taché Y. Nesfatin-1 immunoreactivity in rat brain and spinal cord autonomic nuclei. Neurosci Lett. 2009;452:241–6.
Könczöl K, Bodnar I, Zelena D, Pinter O, Papp RS, Palkovits M, et al. Nesfatin-1/NUCB2 may participate in the activation of the hypothalamic-pituitary-adrenal axis in rats. Neurochem Int. 2010;53:189–97.
Tanida M, Mori M. Nesfatin-1 stimulates renal sympathetic nerve activity in rats. NeuroReport. 2011;2:309–12.
Yosten GLC, Samson WK. The anorexigenic and hypertensive effects of nesfatin-1 are reversed by pretreatment with an oxytocin receptor antagonist. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1642–7.
Okere B, Xu L, Roubos EW, Sonetti D, Kozicz T. Restraint stress alters the secretory activity of neurons co-expressing urocortin-1, cocaine- and amphetamine-regulated transcript peptide and nesfatin-1 in the mouse Edinger-Westphal nucleus. Brain Res. 2010;1317:92–9.
Bonnet MS, Pecchi E, Trouslard J, Jean A, Dallaporta M, Troadec JD. Central nesfatin-1 expressing neurons are sensitive to peripheral imflammatory stimulus. J Neuroinflamm. 2009;6:27.
Yoshida N, Maejima Y, Sedbazar U, Ando A, Kurita H, Damdindorj B, et al. Stressor-responsive central nesfatin-1 activates corticotropin-releasing hormone, noradrenaline and serotonin neurons and evokes hypothalamic-pituitary-adenal axis. Aging. 2010;2:775–84.
Owens MJ, Knight DL, Nemeroff CB. Second-generation SSRIs: human monoamine transporter binding profile of escitalopram and R-fluoxetine. Biol Psychiatry. 2001;50:345–50.
Ranjbar S, Pai N, Deng C. The association of antidepressant medication and body weight gain. Online J Health Allied Sci. 2013;12:1–9.
Okamura N, Habay SA, Zeng J, Chamberlin AR, Reinscheid RK. Synthesis and pharmacological in vitro and in vivo profile of 3-oxo-1,1-diphenyl-tetrahydro-oxazolo[3,4-a]pyrazine-7-carboxylic acid 4-fluoro-benzylamide (SHA68), a selective antagonist of the neuropeptide S receptor. J Pharmacol Exp Ther. 2008;325:893–901.
Shukla AK, Reinhart C, Michel H. Dimethylsulphoxide as a tool to increase functional expression of heterologously produced GPCRs in mammalian cells. FEBS Lett. 2006;580:4261–5.
Xia H, Liu L, Reinhart C, Michel H. Heterologous expression of human neuromedin U receptor 1 and its subsequent solubilization and purification. Biochim Biophys Acta. 2008;1778:2203–9.
Talmont F, Mollereau C, Zajac JM. Expression of opioid and anti-opioid receptors in Chinese hamster ovary cells after exposure to dimethyl sulfoxide. Anal Biochem. 2012;420(1):99–100. https://doi.org/10.1016/j.ab.2011.09.001.
Cavaletti G, Oggioni N, Sala F, Pezzoni G, Cavalletti E, Marmiroli P, et al. Effect on the peripheral nervous system of systemically administered dimethylsulfoxide in the rat: a neurophysiological and pathological study. Toxicol Lett. 2000;118(1–2):103–7. https://doi.org/10.1016/s0378-4274(00)00269-1.
Cavas M, Beltrán D, Navarro JF. Behavioural effects of dimethyl sulfoxide (DMSO): changes in sleep architecture in rats. Toxicol Lett. 2005;157(3):221–32.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8.
Leonard SK, Ring RH. Immunohistochemical localization of the neuropeptide S receptor in the rat central nervous system. Neuroscience. 2011;172:153–63.
Jiang JH, Peng YL, Zhang PJ, Xue HX, He Z, Liang XY, Chang M. The ventromedial hypothalamic nucleus plays an important role in anxiolytic-like effect of neuropeptide S. Neuropeptides. 2018;67:36–44.
Gross CT, Canteras NS. The many paths to fear. Nat Rev Neurosci. 2012;13(9):651–8.
Pringle A, Browning M, Cowen PJ, Harmer CJ. A cognitive neuropsychological model of antidepressant drug action. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(7):1586–92. https://doi.org/10.1016/j.pnpbp.2010.07.022.
Harmer CJ, Goodwin GM, Cowen PJ. Why do antidepressants take so long to work? A cognitive neuropsychological model of anti-depressant drug action. Br J Psychiatry. 2009;195:102–8.
Outhred T, Das P, Felmingham KL, Bryant RA, Nathan PJ, Malhi GS, Kemp AH. Impact of acute administration of escitalopram on the processing of emotional and neutral images: a randomized crossover fMRI study of healthy women. J Psychiatry Neurosci. 2014;39(4):267–75. https://doi.org/10.1503/jpn.130118.
Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206.
Murphy SE, Norbury R, O’Sullivan U, Cowen PJ, Harmer CJ. Effect of a single dose of citalopram on amygdala response to emotional faces. Br J Psychiatry. 2009;194(6):535–40. https://doi.org/10.1192/bjp.bp.108.056093.
Pałasz A, Rojczyk E. Neuroleptics affect neuropeptide S and NPSR mRNA levels in the rat brain. J Mol Neurosci. 2015;57(3):352–7.
Cannella N, Kallupi M, Ruggeri B, Ciccocioppo R, Ubaldi M. The role of the neuropeptide S system in addiction: focus on its interaction with the CRF and hypocretin/orexin neurotransmission. Prog Neurobiol. 2013;100:48–59.
Ubaldi M, Giordano A, Severi I, Li H, Kallupi M, De Guglielmo G, Ruggeri B, et al. Activation of hypocretin-1/orexin-a neurons projecting to the bed nucleus of the stria terminalis and paraventricular nucleus is critical for reinstatement of alcohol seeking by neuropeptide S. Biol Psychiatry. 2016;79:452–62.
Pałasz A, Bandyszewska M, Rojczyk E, Wiaderkiewicz R. Effect of extended olanzapine administration on POMC and neuropeptide Y mRNA levels in the male rat amygdala and hippocampus. Pharmacol Rep. 2016;68:292–6.
Sattin A, Pekary AE, Blood J. Escitalopram regulates expression of TRH and TRH-like peptides in rat brain and peripheral tissues. Neuroendocrinology. 2008;88:135–46.
Kursungoz C, Ak M, Yanik T. Efects of risperidone treatment on the expression of hypothalamic neuropeptide in appetite regulation in Wistar rats. Brain Res. 2015;1596:146–55.
Barone I, Melani R, Mainardi M, Scabia G, Scali M, Dattilo A, et al. Fluoxetine modulates the activity of hypothalamic POMC neurons via mTOR signaling. Mol Neurobiol. 2018;55:9267–79.
Soga T, Wong DW, Clarke IJ, Parhar IS. Citalopram (antidepressant) administration causes sexual dysfunction in male mice through RF-amide related peptide in the dorsomedial hypothalamus. Neuropharmacology. 2010;59:77–85.
Sasaki-Hamada S, Maeno Y, Yabe M, Ishibashi H. Neuromedin U modulates neuronal excitability in rat hippocampal slices. Neuropeptides. 2021;89: 102168.
Kaisho T, Nagai H, Asakawa T, Suzuki N, Fujita H, Matsumiya K, et al. Effects of peripheral administration of a Neuromedin U receptor 2-selective agonist on food intake and body weight in obese mice. Int J Obes (Lond). 2017;41(12):1790–7.
Nakahara K, Katayama T, Maruyama K, Ida T, Mori K, Miyazato M, et al. Comparison of feeding suppression by the anorexigenic hormones neuromedin U and neuromedin S in rats. J Endocrinol. 2010;207:185–93.
McCue DL, Kasper JM, Hommel JD. Regulation of motivation for food by neuromedin U in the paraventricular nucleus and the dorsal raphe nucleus. Int J Obes (Lond). 2017;41(1):120–8.
Benzon C, Johnson S, McCue D, Li D, Green T, Hommel J. Neuromedin U receptor 2 knockdown in the paraventricular nucleus modifies behavioral responses to obesogenic high-fat food and leads to increased body weight. Neuroscience. 2014;258:270–9.
Peng YL, Ha RW, Chang M, Zhang L, Zhang RS, Li W, et al. Central Neuropeptide S inhibits food intake in mice through activation of Neuropeptide S receptor. Peptides. 2010;31:2259–63.
Smith KL, Patterson M, Dhillo WS, Patel SR, Semjonous NM, Gardiner JV, et al. Neuropeptide S stimulates the hypothalamo-pituitary-adrenal axis and inhibits food intake. Endocrinology. 2006;47:3510–8.
Nakahara K, Akagi A, Shimizu S, Tateno S, Qattali AW, Mori K, et al. Involvement of endogenous neuromedin U and neuromedin S in thermoregulation. Biochem Biophys Res Commun. 2016;470(4):930–5.
Ahnaou A, Drinkenburg WH. Neuromedin U(2) receptor signaling mediates alteration of sleep-wake architecture in rats. Neuropeptides. 2011;45(2):165–74.
Pałasz A, Żarczyński P, Bogus K, Mordecka-Chamera K, Della Vecchia A, Skałbania J, et al. Modulatory effect of olanzapine on SMIM20/phoenixin, NPQ/spexin and NUCB2/nesfatin-1 gene expressions in the rat brainstem. Pharmacol Rep. 2021;73(4):1188–94.
Wei Y, Li J, Wang H, Wang G. NUCB2/nesfatin-1: Expression and functions in the regulation of emotion and stress. Prog Neuropsychopharmacol Biol Psychiatry. 2018;81:221–7.
Funding
This work was supported by the Medical University of Silesia grant for the Department of Histology No. PCN-1-011/K/0/O.
Author information
Authors and Affiliations
Contributions
APN, AP, ADV: conceptualization, investigation, data curation, writing—original draft. APN, AP, ASS: Methodology, immunohistochemistry, tissue acquisition. ADV, AG: resources. AP, MK, JJW: formal analysis, corrections.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Piwowarczyk-Nowak, A., Pałasz, A., Suszka-Świtek, A. et al. Escitalopram alters local expression of noncanonical stress-related neuropeptides in the rat brain via NPS receptor signaling. Pharmacol. Rep 74, 637–653 (2022). https://doi.org/10.1007/s43440-022-00374-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-022-00374-z