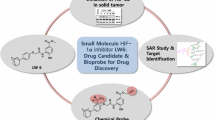
Abstract
Background
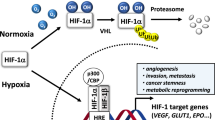
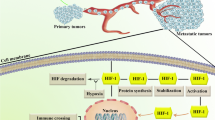
Hypoxic microenvironment is a common feature of solid tumors, which leads to the promotion of cancer. The transcription factor, HIF-1α, expressed under hypoxic conditions stimulates tumor angiogenesis, favoring HIF-1α as a promising anticancer agent. On the other hand, synthetic Indolephenoxyacetamide derivatives are known for their pharmacological potentiality. With this background here, we have synthesized, characterized, and validated the new IPA (8a–n) analogs for anti-tumor activity.
Methods
The new series of IPA (8a–n) were synthesized through a multi-step reaction sequence and characterized based on the different spectroscopic analysis FT-IR, 1H, 13C NMR, mass spectra, and elemental analyses. Cell-based screening of IPA (8a–n) was assessed by MTT assay. Anti-angiogenic efficacy of IPA (8k) validated through CAM, Rat corneal, tube formation and migration assay. The underlying molecular mechanism is validated through zymogram and IB studies. The in vivo anti-tumor activity was measured in the DLA solid tumor model.
Results
Screening for anti-proliferative studies inferred, IPA (8k) is a lead molecule with an IC50 value of ˜5 μM. Anti-angiogenic assays revealed the angiopreventive activity through inhibition of HIF-1α and modulation downstream regulatory genes, VEGF, MMPs, and P53. The results are confirmative in an in vivo solid tumor model.
Conclusion
The IPA (8k) is a potent anti-proliferative molecule with anti-angiogenic activity and specifically targets HIF1α, thereby modulates its downstream regulatory genes both in vitro and in vivo. The study provides scope for new target-specific drug development against HIF-1α for the treatment of solid tumors.
Graphic abstract







Similar content being viewed by others
References
Zimna A, Kurpisz M. Hypoxia-inducible factor-1 in physiological and pathophysiological angiogenesis: applications and therapies. BioMed Res Int. 2015;2015:549412.
Semenza GL. Hypoxia-inducible factor 1 and cardiovascular disease. Annu Rev Physiol. 2014;76:39–65.
Ma Z, Xiang X, Li S, Xie P, Gong Q, Goh BC, et al. Targeting hypoxia-inducible factor-1, for cancer treatment: recent advances in developing small-molecule inhibitors from natural compounds. Semin Cancer Biol. 2020. https://doi.org/10.1016/j.semcancer.2020.09.011.
Fuse S, Suzuki K, Kuchimaru T, Kadonosono T, Ueda H, Sato S, et al. Design, synthesis, and evaluation of indeno [2, 1-c] pyrazolones for use as inhibitors against hypoxia-inducible factor (HIF)-1 transcriptional activity. Bioorganic Med Chem. 2020;28:115207.
Jin X, Dai L, Ma Y, Wang J, Liu Z. Implications of HIF-1 in the tumorigenesis and progression of pancreatic cancer. Cancer Cell Int. 2020;20:1–11.
Gurupadaswamy HD, Thirusangu P, Avin BV, Vigneshwaran V, Kumar MP, Abhishek TS, et al. DAO-9 (2, 5-di (4-aryloylaryloxymethyl)-1, 3, 4-oxadiazole) exhibits p53 induced apoptogenesis through caspase-3 mediated endonuclease activity in murine carcinoma. Biomed Pharmacother. 2014;68:791–7.
Shimazaki Y, Yajima T, Takani M, Yamauchi O. Metal complexes involving indole rings: structures and effects of metal–indole interactions. Coord Chem Rev. 2009;253:479–92.
Demurtas M, Baldisserotto A, Lampronti I, Moi D, Balboni G, Pacifico S, et al. Indole derivatives as multifunctional drugs: synthesis and evaluation of antioxidant, photoprotective and antiproliferative activity of indolehydrazones. Bioorganic Chem. 2019;85:568–76.
Lakshmi NV, Thirumurugan P, Noorulla KM, Perumal PT. InCl3 mediated one-pot multicomponent synthesis, anti-microbial, antioxidant and anticancer evaluation of 3-pyranyl indole derivatives. Bioorganic Med Chem Lett. 2010;20:5054–61.
Merino I, Monge A, Font M, de Irujo JJ, Alberdi E, Santiago E, et al. Synthesis and anti-HIV-1 activities of new pyrimido [5, 4-b] indoles. IlFarmaco. 1999;54:255–64.
Brew CT, Aronchik I, Hsu JC, Sheen JH, Dickson RB, Bjeldanes LF, et al. Indole-3-carbinol activates the ATM signaling pathway independent of DNA damage to stabilize p53 and induce G1 arrest of human mammary epithelial cells. Int J Cancer. 2006;118:857–68.
Meng Q, Qi M, Chen DZ, Yuan R, Goldberg ID, Rosen E, et al. Suppression of breast cancer invasion and migration by indole-3-carbinol: associated with up-regulation of BRCA1 and E-cadherin/catenin complexes. J Mol Med. 2000;78:155–65.
Al-Ostoot FH, Geetha DV, Mohammed YH, Akhileshwari P, Sridhar MA, Khanum SA. Design-based synthesis, molecular docking analysis of an anti-inflammatory drug, and geometrical optimization and interaction energy studies of an indole acetamide derivative. J Mol Struct. 2020;1202:127244.
Al-Ostoot FH, Grisha S, Mohammed YH, Vivek HK, Khanum SA. Molecular docking and synthesis of caffeic acid analogous and its anti-inflammatory, analgesic and ulcerogenic studies. Bioorganic Med Chem Lett. 2021;33:127743.
Khamees HA, Mohammed YH, Swamynayaka A, Al-Ostoot FH, Sert Y, Alghamdi S, et al. Molecular structure, DFT, vibrational spectra with fluorescence effect, hirshfeld surface, docking simulation and antioxidant activity of thiazole derivative. ChemistrySelect. 2019;4:4544–58.
Ranganatha VL, Avin BV, Thirusangu P, Prashanth T, Prabhakar BT, Khanum SA. Synthesis, angiopreventive activity, and in vivo tumor inhibition of novel benzophenone–benzimidazole analogs. Life Sci. 2013;93:904–11.
Vijay Avin BR, Thirusangu P, Ranganatha VL, Firdouse A, Prabhakar BT, Khanum SA. Synthesis and tumor inhibitory activity of novel coumarin analogs targeting angiogenesis and apoptosis. Eur J Med Chem. 2014;75:211–21.
Thirusangu P, Vigneshwaran V, Prashanth T, Avin BV, Malojirao VH, Rakesh H, et al. BP-1T, an antiangiogenic benzophenone-thiazole pharmacophore, counteracts HIF-1 signalling through p53/MDM2-mediated HIF-1? proteasomal degradation. Angiogenesis. 2017;20:55–71.
Vigneshwaran V, Thirusangu P, Vijay Avin BR, Krishna V, Pramod SN, Prabhakar BT. Immunomodulatory glc/man-directed Dolichos lablab lectin (DLL) evokes anti-tumour response in vivo by counteracting angiogenic gene expressions. Clin Exp Immunol. 2017;189:21–35.
Avin BV, Prabhu T, Ramesh CK, Vigneshwaran V, Riaz M, Jayashree K, et al. New role of lupeol in reticence of angiogenesis, the cellular parameter of neoplastic progression in tumorigenesis models through altered gene expression. Biochem Biophys Res Commun. 2014;448:139–44.
Zabiulla Z, Malojirao VH, Mohammed YH, Thirusangu P, Prabhakar BT, Khanum SA. Synthesis, molecular docking, and apoptogenic efficacy of novel N-heterocycle analogs to target B-cell lymphoma 2/X-linked inhibitors of apoptosis proteins to regress melanoma. Med Chem Res. 2019;28:1132–60.
Vigneshwaran V, Thirusangu P, Madhusudana S, Krishna V, Pramod SN, Prabhakar BT. The latex sap of the ‘Old World Plant’Lagenaria siceraria with potent lectin activity mitigates neoplastic malignancy targeting neovasculature and cell death. Int Immunopharmacol. 2016;39:158–71.
Thirusangu P, Vigneshwaran V, Ranganatha VL, Avin BV, Khanum SA, Mahmood R, et al. A tumoural angiogenic gateway blocker, Benzophenone-1B represses the HIF-1α nuclear translocation and its target gene activation against neoplastic progression. Biochem Pharmacol. 2017;125:26–40.
Prabhakar BT, Khanum SA, Shashikanth S, Salimath BP. Antiangiogenic effect of 2-benzoyl–phenoxy acetamide in EAT cell is mediated by HIF-1α and down regulation of VEGF of in-vivo. Invest New Drugs. 2006;24:471–8.
Prabhakar BT, Khanum SA, Jayashree K, Salimath BP, Shashikanth S. Anti-tumor and proapoptotic effect of novel synthetic benzophenone analogues in Ehrlich ascites tumor cells. Bioorganic Med Chem. 2006;14:435–46.
Maj E, Papiernik D, Wietrzyk J. Antiangiogenic cancer treatment: The great discovery and greater complexity. Int J Oncol. 2016;49:1773–84.
Xu D, Xu Z. Indole Alkaloids with potential anticancer activity. Curr Top Med Chem. 2020;20:1938–49.
Mohammed YH, Thirusangu P, Vigneshwaran V, Prabhakar BT, Khanum SA. The anti-invasive role of novel synthesized pyridazine hydrazide appended phenoxy acetic acid against neoplastic development targeting matrix metallo proteases. Biomed Pharmacother. 2017;95:375–86.
Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat Rev Cancer. 2010;10:505–14.
Rogers MS, Birsner AE, D’amato RJ. The mouse cornea micropocket angiogenesis assay. Nat Protoc. 2007;2:2545–50.
Shivakumar S, Prabhakar BT, Jayashree K, Rajan MG, Salimath BP. Evaluation of serum vascular endothelial growth factor (VEGF) and microvessel density (MVD) as prognostic indicators in carcinoma breast. J Cancer Res Clin Oncol. 2009;135:627–36.
Acknowledgements
Fares Hezam Al-Ostoot is thankful to the government of Yemen and Al-Baydha University, Yemen, for providing financial assistance under the teacher's fellowship and also thankful to the University of Mysore, Mysore, India. Ankith Sherapur thanks the Lady Tata Memorial Trust for JRS (2020–2021) and B.T. Prabhakar gratefully acknowledges the grant support by VGST (VGST/P-2/CISEE/GRD-231/2013-14) and SERB [EMR/2017/00088/dated 3/01/2019]. Shaukath Ara Khanum thankfully acknowledges the financial support provided by VGST, Bangalore, under CISEE Program [Project sanction order: No. VGST/CISEE/282/2012-13].
Author information
Authors and Affiliations
Contributions
FHA and AS performed all the experiments, VV and HKV assisted in experiments, GB supported through statistical analysis, SAK and PBT designed the experiments, wrote the manuscript and monitored the entire investigation.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Al-Ostoot, F.H., Sherapura, A., V, V. et al. Targeting HIF-1α by newly synthesized Indolephenoxyacetamide (IPA) analogs to induce anti-angiogenesis-mediated solid tumor suppression. Pharmacol. Rep 73, 1328–1343 (2021). https://doi.org/10.1007/s43440-021-00266-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-021-00266-8