Abstract
Background
Glioblastoma multiforme (GBM), a stage IV astrocytoma, is the most common brain malignancy among adults. Conventional treatments of surgical resection followed by radio and/or chemotherapy fail to completely eradicate the tumor. Resistance to the currently available therapies is mainly attributed to a subpopulation of cancer stem cells (CSCs) present within the tumor bulk that self-renew leading to tumor relapse with time. Therefore, identification of characteristic markers specific to these cells is crucial for the development of targeted therapies. Glycogen synthase kinase 3 (GSK-3), a serine–threonine kinase, is deregulated in a wide range of diseases, including cancer. In GBM, GSK-3β is overexpressed and its suppression in vitro has been shown to induce apoptosis of cancer cells.
Methods
In our study, we assessed the effect of GSK-3β inhibition with Tideglusib (TDG), an irreversible non-ATP competitive inhibitor, using two human GBM cell lines, U-251 MG and U-118 MG. In addition, we combined TDG with radiotherapy to assess whether this inhibition enhances the effect of standard treatment.
Results
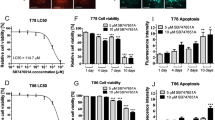
Our results showed that TDG significantly reduced cell proliferation, cell viability, and migration of both GBM cell lines in a dose- and time-dependent manner in vitro. Treatment with TDG alone and in combination with radiation significantly decreased the colony formation of U-251 MG cells and the sphere formation of both cell lines, by targeting and reducing their glioblastoma cancer stem-like cells (GSCs) population. Finally, cells treated with TDG showed an increased level of unrepaired radio-induced DNA damage and, thus, became sensitized toward radiation.
Conclusions
In conclusion, TDG has proven its effectiveness in targeting the cancerous properties of GBM in vitro and may, hence, serve as a potential adjuvant radio-therapeutic agent to better target this deadly tumor.







Similar content being viewed by others
Availability of data and material (data transparency)
All data generated or analyzed during this study were included in this published article.
Change history
04 February 2021
A Correction to this paper has been published: https://doi.org/10.1007/s43440-020-00194-z
References
Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro-oncology. 2014;16(Suppl 4):iv1-63.
Touat M, Idbaih A, Sanson M, Ligon KL. Glioblastoma targeted therapy: updated approaches from recent biological insights. Ann Oncol. 2017;28:1457–72.
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96.
AANS. Brain Tumors. Types of Brain Tumors 2019
Dhermain F. Radiotherapy of high-grade gliomas: current standards and new concepts, innovations in imaging and radiotherapy, and new therapeutic approaches. Chin J Cancer. 2014;33:16–24.
Yao KC, Komata T, Kondo Y, Kanzawa T, Kondo S, Germano IM. Molecular response of human glioblastoma multiforme cells to ionizing radiation: cell cycle arrest, modulation of the expression of cyclin-dependent kinase inhibitors, and autophagy. J Neurosurg. 2003;98:378–84.
Miyashita K, Kawakami K, Nakada M, Mai W, Shakoori A, Fujisawa H, et al. Potential therapeutic effect of glycogen synthase kinase 3beta inhibition against human glioblastoma. Clin Cancer Res. 2009;15:887–97.
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–66.
Stupp R, Dietrich PY, Ostermann Kraljevic S, Pica A, Maillard I, Maeder P, et al. Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J Clin Oncol. 2002;20:1375–82.
Nam JY, de Groot JF. Treatment of glioblastoma. J Oncol Pract. 2017;13:629–38.
Xu HS, Qin XL, Zong HL, He XG, Cao L. Cancer stem cell markers in glioblastoma - an update. Eur Rev Med Pharmacol Sci. 2017;21:3207–11.
Singh SK, Clarke ID, Hide T, Dirks PB. Cancer stem cells in nervous system tumors. Oncogene. 2004;23:7267–73.
Gimple RC, Bhargava S, Dixit D, Rich JN. Glioblastoma stem cells: lessons from the tumor hierarchy in a lethal cancer. Genes Dev. 2019;33:591–609.
Yi Y, Hsieh IY, Huang X, Li J, Zhao W. Glioblastoma stem-like cells: characteristics, microenvironment, and therapy. Front Pharmacol. 2016;7:477.
Mittal S, Pradhan S, Srivastava T. Recent advances in targeted therapy for glioblastoma. Expert Rev Neurother. 2015;15:935–46.
Jalili-Nik M, Sadeghi MM, Mohtashami E, Mollazadeh H, Afshari AR, Sahebkar A. Zerumbone promotes cytotoxicity in human malignant glioblastoma cells through reactive oxygen species (ROS) generation. Oxid Med Cell Longev. 2020;2020:3237983.
Soukhtanloo M, Mohtashami E, Maghrouni A, Mollazadeh H, Mousavi SH, Roshan MK, et al. Natural products as promising targets in glioblastoma multiforme: a focus on NF-κB signaling pathway. Pharmacol Rep. 2020;72:285–95.
Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29:95–102.
Jope RS, Yuskaitis CJ, Beurel E. Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem Res. 2007;32:577–95.
Vashishtha V, Jinghan N. Antagonistic role of GSK3 isoforms in glioma survival. J Cancer. 2018;9:1846–55.
ALZFORUM. Therapeutics-Tideglusib. ALZFORUM; 2019.
Lovestone S, Boada M, Dubois B, Hull M, Rinne JO, Huppertz HJ, et al. A phase II trial of tideglusib in Alzheimer’s disease. J Alzheimer’s Dis. 2015;45:75–88.
Tolosa E, Litvan I, Hoglinger GU, Burn D, Lees A, Andres MV, et al. A phase 2 trial of the GSK-3 inhibitor tideglusib in progressive supranuclear palsy. Mov Disord. 2014;29:470–8.
Martinez A, Gil C, Perez DI. Glycogen synthase kinase 3 inhibitors in the next horizon for Alzheimer’s disease treatment. Int J Alzheimers Dis. 2011;2011:280502.
Dominguez JM, Fuertes A, Orozco L, del Monte-Millan M, Delgado E, Medina M. Evidence for irreversible inhibition of glycogen synthase kinase-3beta by tideglusib. J Biol Chem. 2012;287:893–904.
Mathuram TL, Ravikumar V, Reece LM, Karthik S, Sasikumar CS, Cherian KM. Tideglusib induces apoptosis in human neuroblastoma IMR32 cells, provoking sub-G0/G1 accumulation and ROS generation. Environ Toxicol Pharmacol. 2016;46:194–205.
Mathuram TL, Ravikumar V, Reece LM, Sasikumar CS, Cherian KM. Correlative studies unravelling the possible mechanism of cell death in tideglusib-treated human ovarian teratocarcinoma-derived PA-1 cells. J Environm Pathol Toxicol Oncol. 2017;36:321–44.
Sun A, Li C, Chen R, Huang Y, Chen Q, Cui X, et al. GSK-3beta controls autophagy by modulating LKB1-AMPK pathway in prostate cancer cells. Prostate. 2016;76:172–83.
Westermark B. The deficient density-dependent growth control of human malignant glioma cells and virus-transformed glia-like cells in culture. Int J Cancer. 1973;12:438–51.
Macintyre EH, Pontén J, Vatter AE. The ultrastructure of human and murine astrocytes and of human fibroblasts in culture. Acta pathologica et microbiologica Scandinavica Section A Pathol. 1972;80:267–83.
van Meerloo J, Kaspers GJ, Cloos J. Cell sensitivity assays: the MTT assay. Methods Molecular Biol (Clifton, NJ). 2011;731:237–45.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
Riss TL, Moravec RA, Niles AL, Duellman S, Benink HA, Worzella TJ, et al. Cell Viability Assays. In: Sittampalam GS, Coussens NP, Brimacombe K, Grossman A, Arkin M, Auld D, et al., (eds) Assay Guidance Manual. Bethesda (MD): Eli Lilly & Company and the National Center for Advancing Translational Sciences; 2004.
Strober W. Trypan blue exclusion test of cell viability. Current protocols in immunology. 2001;Appendix 3:Appendix 3B.
Arnold E. Quantifying cell kill and cell survival. Basic Clin Radiobiol London 2009. p. 41–55.
Sart S, Tomasi RFX, Amselem G, Baroud CN. Multiscale cytometry and regulation of 3D cell cultures on a chip. Nature Communic. 2017;8:469.
Abou-Antoun TJ, Hale JS, Lathia JD, Dombrowski SM. Brain cancer stem cells in adults and children: cell biology and therapeutic implications. Neurotherapeutics. 2017;14:372–84.
Weller M, Cloughesy T, Perry JR, Wick W. Standards of care for treatment of recurrent glioblastoma–are we there yet? Neuro-oncology. 2013;15:4–27.
Alifieris C, Trafalis DT. Glioblastoma multiforme: Pathogenesis and treatment. Pharmacol Ther. 2015;152:63–82.
Ghaffari S. Cancer, stem cells and cancer stem cells: old ideas, new developments. F1000 Med Rep. 2011;3:23.
Easton JB, Houghton PJ. mTOR and cancer therapy. Oncogene. 2006;25:6436–46.
Beurel E, Grieco SF, Jope RS. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol Ther. 2015;148:114–31.
Luo J. Glycogen synthase kinase 3beta (GSK3beta) in tumorigenesis and cancer chemotherapy. Cancer Lett. 2009;273:194–200.
Mancinelli R, Carpino G, Petrungaro S, Mammola CL, Tomaipitinca L, Filippini A, et al. Multifaceted roles of GSK-3 in cancer and autophagy-related diseases. Oxid Med Cell Longev. 2017;2017:4629495.
Nakada M. MT, Pyko I, Hayashi Y, Hamada J. The Pivotal Roles of GSK3β in Glioma Biology In: Garami M, (ed) Molecular Targets of CNS Tumors IntechOpen 2011.
Kotliarova S, Pastorino S, Kovell LC, Kotliarov Y, Song H, Zhang W, et al. Glycogen synthase kinase-3 inhibition induces glioma cell death through c-MYC, nuclear factor-kappaB, and glucose regulation. Cancer Res. 2008;68:6643–51.
del Ser T, Steinwachs KC, Gertz HJ, Andres MV, Gomez-Carrillo B, Medina M, et al. Treatment of Alzheimer’s disease with the GSK-3 inhibitor tideglusib: a pilot study. J Alzheimer’s Dis. 2013;33:205–15.
Bahmad HF, Chalhoub RM, Harati H, Bou-Gharios J, Assi S, Ballout F, et al. Tideglusib attenuates growth of neuroblastoma cancer stem/progenitor cells in vitro and in vivo by specifically targeting GSK-3β. Pharmacol Rep. 2020. https://doi.org/10.1007/s43440-020-00162-7.
Foray N, Bourguignon M, Hamada N. Individual response to ionizing radiation. Mutat Res. 2016;770:369–86.
Mori R, Matsuya Y, Yoshii Y, Date H. Estimation of the radiation-induced DNA double-strand breaks number by considering cell cycle and absorbed dose per cell nucleus. J Radiat Res. 2018;59:253–60.
Zhao J, Guo Z, Pei S, Song L, Wang C, Ma J, et al. pATM and γH2AX are effective radiation biomarkers in assessing the radiosensitivity of 12C6+ in human tumor cells. Cancer Cell Int. 2017;17:49.
Bahmad HF, Cheaito K, Chalhoub RM, Hadadeh O, Monzer A, Ballout F, et al. Sphere-formation assay: three-dimensional in vitro culturing of prostate cancer stem/progenitor sphere-forming cells. Front Oncol. 2018;8:347.
Mouhieddine TH, Nokkari A, Itani MM, Chamaa F, Bahmad H, Monzer A, et al. Metformin and ara-a effectively suppress brain cancer by targeting cancer stem/progenitor cells. Front Neurosci. 2015;9:442.
Acknowledgments
We would like to thank all members in the Abou-Kheir’s Laboratory (The WAK Lab) for their help on this work. In addition, we would like to thank members of the core facilities in the DTS Building at the American University of Beirut (AUB) for their help and support.
Funding
This work was supported by the Lebanese National Council for Scientific Research Grant Research Program (LNCSR-GRP) (Grant # 01-10-17; to YF), the Neuroscience Research Center, Faculty of Medicine, Lebanese University (LU) (to HH), and the Medical Practice Plan (MPP) at the American University of Beirut – Faculty of Medicine (AUB-FM) (to WAK). Funders had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
JBG: Formal analysis, Investigation, Methodology, Writing- Original draft preparation. SA: Formal analysis, Investigation, Methodology, Writing—Original draft preparation. HFB: Project administration, Supervision, Formal analysis, Investigation, Methodology, Writing- Reviewing and Editing. HK: Investigation, Methodology, Writing- Reviewing and Editing. TA: Investigation, Methodology, Writing- Reviewing and Editing. RMC: Investigation, Methodology, Writing- Reviewing and Editing, Validation. FB: Investigation, Methodology, Writing- Reviewing and Editing. HH: Resources, Funding acquisition, Writing—Reviewing and Editing, Supervision, Validation, Visualization. YF: Project administration, Funding acquisition, Writing- Reviewing and Editing, Supervision, Validation, Visualization. WAK: Conceptualization, Project administration, Resources, Software, Supervision, Funding acquisition, Writing—Reviewing and Editing, Validation, Visualization.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The spelling of Youssef Fares’ name was incorrect.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bou-Gharios, J., Assi, S., Bahmad, H.F. et al. The potential use of tideglusib as an adjuvant radio-therapeutic treatment for glioblastoma multiforme cancer stem-like cells. Pharmacol. Rep 73, 227–239 (2021). https://doi.org/10.1007/s43440-020-00180-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-020-00180-5