Abstract
Background
Although several studies had addressed the anti-inflammatory effects of derivatives of 4H-chromene and chromeno[2,3-b]pyridine in the different types of cells, whether these derivatives would exert beneficial anti-fibrotic effects during corneal fibrotic scar formation was unclear.
Methods
We examined the cyclooxygenase-2 (COX-2) expression of 2,4-diamino-5-(1-hydroxynaphthalen-2-yl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile (N1) in the human corneal fibroblasts (HCFs) under the treatment TGF-β1. Signaling pathways underlying the mechanism of the N1 effect on the HCFs were determined.
Results
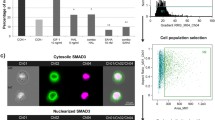
Application of N1 significantly decreased COX-2 expression after 2 h and 4 h in the HCFs stimulated with TGF-β1. Notably, reduced production of extracellular matrix proteins under N1 treatment was found, including fibronectin, collagen I, and matrix metallopeptidase 9. Immunoblot analysis showed that treatment with N1 significantly attenuated phosphorylation of both STAT3 and Smad 2 in the TGF-β1-stimulated HCFs. Upregulated mRNA of Smad2 and downregulated mRNA of Smad3 were observed using the quantitative real-time polymerase chain reaction. In addition, N1 induced significant increases in HO-1 and Nrf2 expression, but inhibited phosphorylation of NF-κB in the HCFs treated with TGF-β1.
Conclusions
Our findings show for the first time that N1 exerts anti-fibrotic and anti-inflammatory effects through suppression of COX-2, Smad2, STAT3, iNOS and NF-κB expressions as well as upregulation of Nrf2 and HO-1 expressions, which suggests they are potential therapeutic targets in the treatment of corneal fibrosis.







Similar content being viewed by others
Abbreviations
- COX-2:
-
Cyclooxygenase-2
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- EGF:
-
Epidermal growth factor
- FGF:
-
Fibroblast growth factor
- FBS:
-
Fetal bovine serum
- HCF:
-
Human corneal fibroblast
- iNOS:
-
Inducible nitric oxide synthase
- IGF-I:
-
Insulin-like growth factor I
- KGF:
-
Keratinocyte growth factor
- MMP-9:
-
Matrix metallopeptidase 9
- MAPK:
-
p38 Mitogen-activated protein kinase
- N1:
-
2,4-Diamino-5-(1-hydroxynaphthalen-2-yl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile
- PDGF:
-
Platelet-derived growth factor
- PCR:
-
Polymerase chain reaction
- TGF:
-
Transforming growth factor
References
Newsome DA, Gross J, Hassell JR. Human corneal stroma contains three distinct collagens. Invest Ophthalmol Vis Sci. 1982;22:376–81.
Meek KM, Boote C. The use of X-ray scattering techniques to quantify the orientation and distribution of collagen in the corneal stroma. Prog Retin Eye Res. 2009;28:369–92.
Ljubimov AV, Saghizadeh M. Progress in corneal wound healing. Prog Retin Eye Res. 2015;49:17–45.
Sellers RS, Silverman L, Khan KN. Cyclooxygenase-2 expression in the cornea of dogs with keratitis. Vet Pathol. 2004;41:116–21.
Yamada M, Kawai M, Kawai Y, Mashima Y. The effect of selective cyclooxygenase-2 inhibitor on corneal angiogenesis in the rat. Curr Eye Res. 1999;19:300–4.
Kemnitzer W, Drewe J, Jiang S, Zhang H, Crogan-Grundy C, Labreque D, et al. Discovery of 4-aryl-4H-chromenes as a new series of apoptosis inducers using a cell- and caspase-based high throughput screening assay. 4. Structure-activity relationships of N-alkyl substituted pyrrole fused at the 7,8-positions. J Med Chem. 2008;51:417–23.
Shestopalov AM, Litvinov YM, Rodinovskaya LA, Malyshev OR, Semenova MN, Semenov VV. Polyalkoxy substituted 4H-chromenes: synthesis by domino reaction and anticancer activity. ACS Comb Sci. 2012;14:484–90.
Akyol-Salman I, Lece-Sertoz D, Baykal O. Topical pranoprofen 0.1% is as effective anti-inflammatory and analgesic agent as diclofenac sodium 0.1% after strabismus surgery. J Ocul Pharmacol Ther. 2007;23:280–3.
Ahuja M, Dhake AS, Sharma SK, Majumdar DK. Topical ocular delivery of NSAIDs. AAPS J. 2008;10:229–41.
Wu J, Du Y, Mann MM, Funderburgh JL, Wagner WR. Corneal stromal stem cells versus corneal fibroblasts in generating structurally appropriate corneal stromal tissue. Exp Eye Res. 2014;120:71–81.
Chen TC, Chang SW. Moxifloxacin modulated TGF-beta1-related Interleukin-12 secretion in corneal fibroblasts. Invest Ophthalmol Vis Sci. 2017;58:5692–702.
Das SK, Gupta I, Cho YK, Zhang X, Uehara H, Muddana SK, et al. Vimentin knockdown decreases corneal opacity. Invest Ophthalmol Vis Sci. 2014;55:4030–40.
Lee C, Liao J, Chen S, Yen C, Lee Y, Huang S, et al. Fluorine-modified rutaecarpine exerts cyclooxygenase-2 inhibition and anti-inflammatory effects in lungs. Front Pharmacol. 2019;10:91.
Chung S, Huang WH, Huang CK, Liu FC, Huang RY, Wu CC, Lee AR. Synthesis and anti-inflammatory activities of 4H-chromene and chromeno[2,3-b]pyridine derivatives. Res Chem Intermed. 2016;42:1195–215.
Horvath CM. STAT proteins and transcriptional responses to extracellular signals. Trends Biochem Sci. 2000;25:496–502.
Fuller GM, Zhang Z. Transcriptional control mechanism of fibrinogen gene expression. Ann N Y Acad Sci. 2001;936:469–79.
Ogata H, Chinen T, Yoshida T, Kinjyo I, Takaesu G, Shiraishi H, et al. Loss of SOCS3 in the liver promotes fibrosis by enhancing STAT3-mediated TGF-beta1 production. Oncogene. 2006;25:2520–30.
Pang M, Ma L, Gong R, Tolbert E, Mao H, Ponnusamy M, et al. A novel STAT3 inhibitor, S3I-201, attenuates renal interstitial fibroblast activation and interstitial fibrosis in obstructive nephropathy. Kidney Int. 2010;78:257–68.
Park J, Lee SY, Ooshima A, Yang KM, Kang JM, Kim YW, et al. Glucosamine hydrochloride exerts a protective effect against unilateral ureteral obstruction-induced renal fibrosis by attenuating TGF-beta signaling. J Mol Med (Berl). 2013;91:1273–84.
Honma S, Shinohara M, Takahashi N, Nakamura K, Hamano S, Mitazaki S, et al. Effect of cyclooxygenase (COX)-2 inhibition on mouse renal interstitial fibrosis. Eur J Pharmacol. 2014;740:578–83.
Nunez O, Fernandez-Martinez A, Majano PL, Apolinario A, Gomez-Gonzalo M, Benedicto I, et al. Increased intrahepatic cyclooxygenase 2, matrix metalloproteinase 2, and matrix metalloproteinase 9 expression is associated with progressive liver disease in chronic hepatitis C virus infection: role of viral core and NS5A proteins. Gut. 2004;53:1665–72.
Zhang R, Liu Z, Zhang H, Zhang Y, Lin D. The COX-2-selective antagonist (NS-398) inhibits choroidal neovascularization and subretinal fibrosis. PLoS One. 2016;11:e0146808.
Huang C, Wang H, Pan J, Zhou D, Chen W, Li W, et al. Benzalkonium chloride induces subconjunctival fibrosis through the COX-2-modulated activation of a TGF-beta1/Smad3 signaling pathway. Invest Ophthalmol Vis Sci. 2014;55:8111–22.
Tian J, Hachim MY, Hachim IY, Dai M, Lo C, Raffa FA, et al. Cyclooxygenase-2 regulates TGFbeta-induced cancer stemness in triple-negative breast cancer. Sci Rep. 2017;7:40258.
Kuo YH, Chen CW, Chu Y, Lin P, Chiang HM. In vitro and in vivo studies on protective action of N-phenethyl caffeamide against photodamage of skin. PLoS One. 2015;10:e0136777.
Gong J, Xie J, Bedolla R, Rivas P, Chakravarthy D, Freeman JW, et al. Combined targeting of STAT3/NF-kappaB/COX-2/EP4 for effective management of pancreatic cancer. Clin Cancer Res. 2014;20:1259–73.
He G, Karin M. NF-kappaB and STAT3—key players in liver inflammation and cancer. Cell Res. 2011;21:159–68.
Liu Y, Liu H, Meyer C, Li J, Nadalin S, Konigsrainer A, et al. Transforming growth factor-beta (TGF-beta)-mediated connective tissue growth factor (CTGF) expression in hepatic stellate cells requires Stat3 signaling activation. J Biol Chem. 2013;288:30708–19.
Tang LY, Heller M, Meng Z, Yu LR, Tang Y, Zhou M, et al. Transforming growth factor-beta (TGF-beta) directly activates the JAK1-STAT3 Axis to induce hepatic fibrosis in coordination with the SMAD pathway. J Biol Chem. 2017;292:4302–12.
Kundu J, Kim DH, Chae IG, Lee JK, Lee S, Jeong CH, et al. Silicon dioxide nanoparticles induce COX-2 expression through activation of STAT3 signaling pathway in HaCaT cells. Toxicol In Vitro. 2018;52:235–42.
Chun KS, Surh YJ. Signal transduction pathways regulating cyclooxygenase-2 expression: potential molecular targets for chemoprevention. Biochem Pharmacol. 2004;68:1089–100.
Neil JR, Johnson KM, Nemenoff RA, Schiemann WP. Cox-2 inactivates Smad signaling and enhances EMT stimulated by TGF-beta through a PGE2-dependent mechanisms. Carcinogenesis. 2008;29:2227–35.
Khalil H, Kanisicak O, Prasad V, Correll RN, Fu X, Schips T, et al. Fibroblast-specific TGF-beta-Smad2/3 signaling underlies cardiac fibrosis. J Clin Invest. 2017;127:3770–83.
Guo X, Ramirez A, Waddell DS, Li Z, Liu X, Wang XF. Axin and GSK3- control Smad3 protein stability and modulate TGF- signaling. Genes Dev. 2008;22:106–20.
Hua X, Chi W, Su L, Li J, Zhang Z, Yuan X. ROS-induced oxidative injury involved in pathogenesis of fungal keratitis via p38 MAPK activation. Sci Rep. 2017;7:10421.
Singer CA, Baker KJ, McCaffrey A, AuCoin DP, Dechert MA, Gerthoffer WT. p38 MAPK and NF-kappaB mediate COX-2 expression in human airway myocytes. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1087–98.
Chi X, Yao W, Xia H, Jin Y, Li X, Cai J, et al. Elevation of HO-1 expression mitigates intestinal ischemia-reperfusion injury and restores tight junction function in a rat liver transplantation model. Oxid Med Cell Longev. 2015;2015:986075.
Lundvig DM, Immenschuh S, Wagener FA. Heme oxygenase, inflammation, and fibrosis: the good, the bad, and the ugly? Front Pharmacol. 2012;3:81.
Ahmed SM, Luo L, Namani A, Wang XJ, Tang X. Nrf2 signaling pathway: pivotal roles in inflammation. Biochim Biophys Acta. 2017;1863:585–97.
Ni HM, Woolbright BL, Williams J, Copple B, Cui W, Luyendyk JP, et al. Nrf2 promotes the development of fibrosis and tumorigenesis in mice with defective hepatic autophagy. J Hepatol. 2014;61:617–25.
Funding
This study was supported in part by grants from the Tri-Service General Hospital (TSGH-C 108-220) and the Ministry of Science and Technology (MOST 107-2314-B-016-035), Taiwan, ROC.
Author information
Authors and Affiliations
Contributions
Conceived and designed the analysis: YJC, SMH, MCT, JTC, CML. Collected the data: YJC, SMH, MCT, JTC, ARL, RYH, CML. Performed the analysis: YJC, SMH, MCT, JTC, CML. Wrote the paper: YJC. Contributed analysis tools: YJC, SMH, MCT, JTC, ARL, RYH, CML.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
43440_2019_26_MOESM1_ESM.pdf
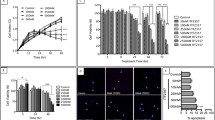
Supplementary Figure S1. In vitro characterization of HCFs (A) Cellular morphology of different participants by microscopy. (B) Immunoblotting analysis showed expression of vimentin in the HCFs of different participants. (C) COX-2 activity was measured with or without N1 (10 nM) and TGF β1 (10 ng/mL) stimulation. (PDF 564 kb)
Rights and permissions
About this article
Cite this article
Chen, YJ., Huang, SM., Tai, MC. et al. The anti-fibrotic and anti-inflammatory effects of 2,4-diamino-5-(1-hydroxynaphthalen-2-yl)-5H-chromeno[2,3-b] pyriine-3-carbonitrile in corneal fibroblasts. Pharmacol. Rep 72, 115–125 (2020). https://doi.org/10.1007/s43440-019-00026-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-019-00026-9