Abstract
Background
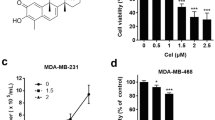
Cleistanthin A (CleA), a natural diphyllin glycoside, has been shown to suppress the invasion of cancer cells, but the underlying mechanisms remain unclear. Here, the inhibitory effect of CleA on the invasion of MDA-MB-231 human breast cancer cells was investigated, and the mechanisms involved were clarified.
Methods
Cell viability was studied by MTT assay. The migration and invasion of MDA-MB-231 cells were assessed by wound healing assay and transwell assay, respectively. The enzymatic activity of matrix metalloproteinases (MMPs) was detected by gelatin zymography. mRNA and protein levels were detected by qRT-PCR and Western blotting, respectively. Nuclear translocation of β-catenin was observed by immunofluorescence and detected by Western blotting.
Results
CleA effectively inhibited the migration and invasion of MDA-MB-231 cells and suppressed the expression and activation of MMP-2/9. Moreover, the expression and nuclear translocation of β-catenin were reduced by CleA treatment, as well as transcription of the Cyclin D1 and c-myc genes. In addition, the inhibitory effect of CleA on the β-catenin pathway was attributed to the promotion of β-catenin degradation by inhibition of GSK3β phosphorylation. When the phosphorylation of GSK3β was induced by LiCl, the inhibitory effect of CleA on the β-catenin pathway and the invasion of MDA-MB-231 cells were almost reversed.
Conclusion
CleA suppressed the invasion of MDA-MB-231 cells, likely through the β-catenin pathway.






Similar content being viewed by others
References
Pradheepkumar CP, Panneerselvam N, Shanmugam G. Cleistanthin A causes DNA strand breaks and induces apoptosis in cultured cells. Mutat Res. 2000;464:185–93.
Parasuraman S, Raveendran R, Rajesh NG, Nandhakumar S. Sub-chronic toxicological evaluation of cleistanthin A and cleistanthin B from the leaves of Cleistanthus collinus (Roxb.). Toxicol Rep. 2014;1:596–611.
Parasuraman S, Raveendran R, Ardestani MS, Ananthakrishnan R, Jabbari-Arabzadeh A, Alavidjeh MS, et al. Biodistribution properties of cleistanthin A and cleistanthin B using magnetic resonance imaging in a normal and tumoric animal model. Pharmacogn Mag. 2012;8:129–34.
Pradheepkumar CP, Shanmugam G. Anticancer potential of cleistanthin A isolated from the tropical plant Cleistanthus collinus. Oncol Res. 1999;11:225–32.
Prabhakaran C, Kumar P, Panneerselvam N, Rajesh S, Shanmugam G. Cytotoxic and genotoxic effects of cleistanthin B in normal and tumour cells. Mutagenesis. 1996;11:553–7.
Zhao Y, Zhang R, Lu Y, Ma J, Zhu L. Synthesis and bioevaluation of heterocyclic derivatives of Cleistanthin-A. Bioorg Med Chem. 2015;23:4884–90.
Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–84.
Pan S, Cai H, Gu L, Cao S. Cleistanthin A inhibits the invasion and metastasis of human melanoma cells by inhibiting the expression of matrix metallopeptidase-2 and -9. Oncol Lett. 2017;14:6217–23.
Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–205.
Cruciat CM, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, Ingelfinger D, et al. Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science. 2010;327:459–63.
Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–98.
Zhang Z, Ma J, Zhu L, Zhao Y. Synthesis and identification of cytotoxic diphyllin glycosides as vacuolar H(+)-ATPase inhibitors. Eur J Med Chem. 2014;82:466–71.
Wang L, Ling Y, Chen Y, Li CL, Feng F, You QD, et al. Flavonoid baicalein suppresses adhesion, migration and invasion of MDA-MB-231 human breast cancer cells. Cancer Lett. 2010;297:42–8.
Albini A, D’Agostini F, Giunciuglio D, Paglieri I, Balansky R, De Flora S. Inhibition of invasion, gelatinase activity, tumor take and metastasis of malignant cells by N-acetylcysteine. Int J Cancer. 1995;61:121–9.
Yang J, Wei D, Wang W, Shen B, Xu S, Cao Y. TRAF4 enhances oral squamous cell carcinoma cell growth, invasion and migration by Wnt-beta-catenin signaling pathway. Int J Clin Exp Pathol. 2015;8:11837–46.
Zhao Y, Ni C, Zhang Y, Zhu L. Synthesis and bioevaluation of diphyllin glycosides as novel anticancer agents. Arch Pharm (Weinheim). 2012;345:622–8.
Teplova VV, Tonshin AA, Grigoriev PA, Saris NE, Salkinoja-Salonen MS. Bafilomycin A1 is a potassium ionophore that impairs mitochondrial functions. J Bioenerg Biomembr. 2007;39:321–9.
von Schwarzenberg K, Wiedmann RM, Oak P, Schulz S, Zischka H, Wanner G, et al. Mode of cell death induction by pharmacological vacuolar H+-ATPase (V-ATPase) inhibition. J Biol Chem. 2013;288:1385–96.
Prasad CP, Manchanda M, Mohapatra P, Andersson T. WNT5A as a therapeutic target in breast cancer. Cancer Metastasis Rev. 2018;37:767–78.
Irshad I, Varamini P. Different targeting strategies for treating breast cancer bone metastases. Curr Pharm Des. 2018;24:3320–31.
Zhang F, Wang Z, Fan Y, Xu Q, Ji W, Tian R, et al. Elevated STAT3 signaling-mediated upregulation of MMP-2/9 confers enhanced invasion ability in multidrug-resistant breast cancer cells. Int J Mol Sci. 2015;16:24772–90.
Zhan Y, Zhang H, Li J, Zhang Y, Zhang J, He L. A novel biphenyl urea derivate inhibits the invasion of breast cancer through the modulation of CXCR4. J Cell Mol Med. 2015;19:1614–23.
Yang F, Hu M, Lei Q, Xia Y, Zhu Y, Song X, et al. Nifuroxazide induces apoptosis and impairs pulmonary metastasis in breast cancer model. Cell Death Dis. 2015;6:e1701.
Song N, Zhong J, Hu Q, Gu T, Yang B, Zhang J, et al. FGF18 enhances migration and the epithelial-mesenchymal transition in breast cancer by regulating Akt/GSK3beta/beta-catenin signaling. Cell Physiol Biochem. 2018;49:1019–32.
Fan SH, Wang YY, Lu J, Zheng YL, Wu DM, Zhang ZF, et al. CERS2 suppresses tumor cell invasion and is associated with decreased V-ATPase and MMP-2/MMP-9 activities in breast cancer. J Cell Biochem. 2015;116:502–13.
Rao JS. Molecular mechanisms of glioma invasiveness: the role of proteases. Nat Rev Cancer. 2003;3:489–501.
Ninomiya I, Endo Y, Fushida S, Sasagawa T, Miyashita T, Fujimura T, et al. Alteration of beta-catenin expression in esophageal squamous-cell carcinoma. Int J Cancer. 2000;85:757–61.
Osterheld MC, Bian YS, Bosman FT, Benhattar J, Fontolliet C. Beta-catenin expression and its association with prognostic factors in adenocarcinoma developed in Barrett esophagus. Am J Clin Pathol. 2002;117:451–6.
Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, et al. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–7.
Zhang P, Zhu D, Chen X, Li Y, Li N, Gao Q, et al. Prenatal hypoxia promotes atherosclerosis via vascular inflammation in the offspring rats. Atherosclerosis. 2016;245:28–34.
Valenta T, Hausmann G, Basler K. The many faces and functions of beta-catenin. EMBO J. 2012;31:2714–36.
Jo M, Lester RD, Montel V, Eastman B, Takimoto S, Gonias SL. Reversibility of epithelial-mesenchymal transition (EMT) induced in breast cancer cells by activation of urokinase receptor-dependent cell signaling. J Biol Chem. 2009;284:22825–33.
Baryawno N, Sveinbjornsson B, Eksborg S, Chen CS, Kogner P, Johnsen JI. Small-molecule inhibitors of phosphatidylinositol 3-kinase/Akt signaling inhibit Wnt/beta-catenin pathway cross-talk and suppress medulloblastoma growth. Can Res. 2010;70:266–76.
Yang S, Liu Y, Li MY, Ng CSH, Yang SL, Wang S, et al. FOXP3 promotes tumor growth and metastasis by activating Wnt/beta-catenin signaling pathway and EMT in non-small cell lung cancer. Mol Cancer. 2017;16:124.
Shi X, Zhu M, Kang Y, Yang T, Chen X, Zhang Y. Wnt/beta-catenin signaling pathway is involved in regulating the migration by an effective natural compound brucine in LoVo cells. Phytomedicine. 2018;46:85–92.
Luo Y, Sun Z, Li Y, Liu L, Cai X, Li Z. Caudatin inhibits human hepatoma cell growth and metastasis through modulation of the Wnt/beta-catenin pathway. Oncol Rep. 2013;30:2923–8.
Chen YZ, Sun DQ, Zheng Y, Zheng GK, Chen RQ, Lin M, et al. WISP1 silencing confers protection against epithelial-mesenchymal transition of renal tubular epithelial cells in rats via inactivation of the Wnt/beta-catenin signaling pathway in uremia. J Cell Physiol. 2019;234:9673–86.
Henderson BR, Fagotto F. The ins and outs of APC and beta-catenin nuclear transport. EMBO Rep. 2002;3:834–9.
Zeng FY, Dong H, Cui J, Liu L, Chen T. Glycogen synthase kinase 3 regulates PAX3-FKHR-mediated cell proliferation in human alveolar rhabdomyosarcoma cells. Biochem Biophys Res Commun. 2010;391:1049–55.
Zheng H, Saito H, Masuda S, Yang X, Takano Y. Phosphorylated GSK3beta-ser9 and EGFR are good prognostic factors for lung carcinomas. Anticancer Res. 2007;27:3561–9.
Tejeda-Munoz N, Robles-Flores M. Glycogen synthase kinase 3 in Wnt signaling pathway and cancer. IUBMB Life. 2015;67:914–22.
Farago M, Dominguez I, Landesman-Bollag E, Xu X, Rosner A, Cardiff RD, et al. Kinase-inactive glycogen synthase kinase 3beta promotes Wnt signaling and mammary tumorigenesis. Can Res. 2005;65:5792–801.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31671206, 31500965, 31471141, and 81702874). We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, S., Wang, L., Ding, W. et al. Cleistanthin A inhibits the invasion of MDA-MB-231 human breast cancer cells: involvement of the β-catenin pathway. Pharmacol. Rep 72, 188–198 (2020). https://doi.org/10.1007/s43440-019-00012-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-019-00012-1