Abstract
Introduction
Probiotic foods, generally dairy-based, aren’t widely affordable in low-income countries. So it’s necessary to suggest suitable and accessible matrices for delivering probiotics.
Objectives
This study aimed to assess the probiotic traits of Lactiplantibacillus plantarum LO3 and its use in the development of a golden apple-based non-dairy probiotic beverage.
Methods
To this end, the probiotic and safety properties of Lact. plantarum LO3 were evaluated. Then, Lact. plantarum LO3 suspension was added to GaJ (≈ log 7.67 cfu) and stored at 4 and 30 °C. During storage, the proximate composition, the DPPH° activity as well as a sensory evaluation of the juice were performed.
Results
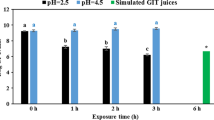
As a result, Lact. plantarum LO3 has excellent viability (˃97%) in gastric and intestinal juices respectively, after 2 and 4 h. As for adhesive properties, the highest co-aggregations were recorded against Escherichia coli (23.43%) and Vibrio parahaemolyticus (22.05%). In the GaJ, except day 21, Lact. plantarum LO3 load was significantly (p˂0.05) higher than the initial load. Ascorbic acid content decreased over time, with minima recorded on day 30 (22.41 and 25.00 mg/100 ml) at 30 and 4 °C respectively. Furthermore, the highest DPPH° activities (EC50) were 90.05 and 94.56 µg/ml at 4 and 30 °C respectively. Carbohydrates and fibre contents decreased significantly (p˂0.05) with storage temperature. In terms of sensory attributes, Lact. plantarum LO3 had a positive effect on odour at 30 °C, while colour was better preserved at 4 °C.
Conclusions
This makes golden apple juice a suitable matrix for carrying the probiotic strain Lact. plantarum LO3 for consumers from the whole spectrum of social classes.


Similar content being viewed by others
References
Syiemlieh I, Morya S. Dairy and non-dairy based probiotics: a review. Pharma Innov J. 2022;11:2956–64.
Khan FM, Morya S, Chattu VK. Probiotics as a boon in food diligence emphasizing the therapeutical role of probiotic beverages on consumers health. J Appl Nat Sci. 2021;13(2):700–14.
Martirosyan DM, Singh J. A new definition of functional food by FFC: what makes a new definition unique? Funct Foods Health Dis. 2015;5(6):209–23.
Parichat P, Pongsak R, Probiotics. Sources, selection and health benefits. Res J Biotechnol. 2023;18(5):102–13.
Mostafidi M, Sanjabi MR, Shirkhan F, Zahedi MT. A review of recent trends in the development of the Microbial Safety of fruits and vegetables. Trends Food Sci Technol. 2020;103:321–32.
FAO/WHO Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food. (2002) Available online: https://www.foodinprogress.com/wp-content/uploads/2019/04/Guidelines-for-the-Evaluation-of-Probioticsin Food.pdf (accessed on 20 January 2023).
Turkmen N, Akal C, Özer B. Probiotic dairy-based beverages: a review. J Funct Foods. 2019;53:62–75.
Rozenberg S, Body J-J, Bruyère O, Bergmann P, Brandi ML, Cooper C, Devogelaer J-P, Gielen E, Goemaere S, Kaufman J-M, Rizzoli R, Reginster J-Y. Effects of dairy products consumption on Health: benefits and Beliefs—A Commentary from the Belgian bone Club and the European Society for Clinical and Economic Aspects of Osteoporosis. Osteoarthritis and Musculoskeletal diseases. Calcif Tissue Int. 2016;98:1–17. D.
FAO/WHO Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food. Available online: https://www.foodinprogress.com/wp-content/uploads/2019/04/Guidelines-for-the-Evaluation-of-Probioticsin- Food.pdf 2002; (accessed on 20 November 2023).
Naseem Z, Mir SA, Wani SM, Rouf MA, Bashir I, Zehra A. Probiotic-fortified fruit juices: Health benefits. Challenges, and future perspective. Nutrition. 2023;115:112154.
Foko KEM, Kaktcham PM, Maffo B, Tchamani PL, Fotso TUD, Zambou NF. Development of a non-dairy probiotic beverage based on sorrel and pineapple juices using lacticaseibacillus paracasei 62L. J Agric Food Res. 2023;14:100688.
Guedes CKRDM, Guedes AFLDM, Silva EBBD, Santos ECMD, Stamford TCM, Stamford TLM. Development of vegetal probiotic beverage of passion fruit (Passiflora edulis Sims), yam (Dioscorea cayenensis) and Lacticaseibacillus casei. Food Sci Technol. 2021; 41:619–626.
de Oliveira Vieira KC, Ferreira CDS, Bueno EBT, De Moraes YA, Toledo ACCG, Nakagaki WR, Winkelstroter LK. Development and viability of probiotic orange juice supplemented by Pediococcus acidilactici CE51. LWT Food Sci Technol. 2020;130:109637.
Furtado LL, Martins ML, Ramos AM, da Silva RR, Junior BRDCL, Martins EMF. Viability of probiotic bacteria in tropical mango juice and the resistance of the strains to gastrointestinal conditions simulated in vitro. Semin Cienc Agrar. 2019;40(1):149–62.
Worku KF, Kurabachew H, Hassen Y. Probiotication of Fruit Juices by Supplemented Culture of Lactobacillus acidophilus. Int J Food Sci Nutr Eng. 2019;9:45–8.
Acevedo-Martínez E, Gutiérrez-Cortés C, García-Mahecha M, Díaz-Moreno C. Evaluation of viability of probiotic bacteria in mango (Mangifera indica L. Cv.Tommy Atkins) beverage. Dyna. 2018;85:84–92.
Mishra A, Athmaselvi K. Stress tolerance and Physicochemical properties of Encapsulation processes for Lactobacillus rhamnosus in Pomegranate (Punica granatum L.) Fruit Juice. Food Sci Biotechnol. 2016;25:125–9.
Jayarathna PLI, Jayawardena JAEC, Vanniarachchy MPG. Identification of physical. Chemical Properties and Flavor Profile of Spondias dulcis in three maturity stages. Int Res J Adv Eng Sci. 2020;5(1):208–11.
Pimentel TC. Fruit juices as probiotic carriers. J Plant Biotechnol Microbiol. 2017;1(1):8–10.
da Costa GM, de Carvalho Silva JV, Mingotti JD, Barão CE, Klososki SJ, Pimentel TC. Effect of ascorbic acid or oligofructose supplementation on L. Paracasei viability, physicochemical characteristics and acceptance of probiotic orange juice. LWT Food Sci Technol. 2017;75:195–201.
White J, Hekmat S. Development of probiotic fruit juices using Lactobacillus rhamnosus GR-1 fortified with short chain and long chain inulin fiber. Fermentation. 2018;4(2):27.
Ribeiro ESS, Damasceno KSFSC, Dantas LMdC, Azevedo WMd, Leite PIP, Assis CFd, de Sousa FCJ. Fermented yellow mombin juice using Lactobacillus acidophilus NRRL B-4495: chemical composition, bioactive properties and survival in simulated gastrointestinal conditions. PLoS ONE. 2020;15(9):e0239392.
Basnayake BSGM, Jemziya MBF, Rambodagalla RTB, Rikasa AM. Development of Ambarella (Spondias dulsis) fruit pulp incorporated ice cream. SLJoT. 2022; 39–43.
Graham OS, Wickham LD, Mohammed M. Growth, development and quality attributes of miniature golden apple fruit (Spondias cytherea Sonn) Part I: Fruit growth and development to maturity. Int J Food Agric Environ. 2004;2(1):90–4.
Turpin W, Humblot. Guyot CJP. Genetic screening of functional properties of lactic acid bacteria in a fermented pearl millet slurry and in the metagenome of fermented starchy foods. Appl Environ Microbiol. 2011;77(24):8722–34. https://doi.org/10.1128/AEM.05988-11.
Kaktcham PM, Zambou NF, Foko KEM, Ciobotaru O, Matei F, Cornea PC, Israel-Roming F. Antifungal activity of lactic acid Bacteria isolated from Peanuts, Gari, and Orange Fruit Juice against Food Aflatoxigenic Molds. Food Biotechnol. 2018;32(4):237–56. https://doi.org/10.1080/08905436.2018.1519443.
Verdenelli MC, Ghelfi F, Silvi S, Orpianesi C, Cecchini C, Cresci A. Probiotic properties of Lactobacillus rhamnosus and Lactobacillus paracasei isolated from human faeces. Eur J Nutr. 2009;48(6):355–63.
Corcoran BM, Stanton C, Fitzgerald GF, Ross RP. Survival of probiotic lactobacilli in acidic environments is enhanced in the presence of metabolizable sugars. Appl Environ Microbiol. 2005;71:3060–7.
Sahoo TK, Jena PK, Nagar N, Patel AK, Seshadri S. In Vitro evaluation of probiotic properties of lactic acid bacteria fromthe gut of Labeo rohita and Catla catla. Probiotics Antimicrob Proteins. 2015. https://doi.org/10.1007/s12602-015-9184-8.
Ekmekci H, Aslim B, Ozturk S. Characterization of vaginal lactobacilli coaggregation ability with Escherichia coli. Microbiol Immunol. 2009;53:59–65.
ISO 10932/IDF 223. Milk and milk products—determination of minimal inhibitory concentration (MIC) of antibiotics applicable to bifidobacteria and non-enterococcal lactic acid bacteria (LAB). 2010.
EFSA. Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 2012;10:2740.
Danielsen M, Wind AA. Susceptibility of Lactobacillus ssp. to antimicrobial agents. Int J Food Microbiol. 2003;82:1–11.
CLSI (Clinical and Laboratory Standards Institute). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved standard-Eight Edition. CLSI document M07-A8 (ISBN 1-56238-689-1). Clinical and Laboratory Standards Institute. 940 West Valley Road, suite 1400. Wayne. Pennsylvania ASA. 2009; 19087–1898.
Gerhardt P, Murray RG, Costilow RN, Nester EW, Wood WA, Krieg NR, Phillips GB. Manual of methos for general bacteriology. American Soc Microbiol Washington. DC 20006. 1981.
Harrigan WF, Mc Cance ME. Laboratory methods. In: Harigan WF, Mc Cance ME, editors. Food and dairy microbiology. New York: Academic; 1990. pp. 12–5.
Bover-Cid S, Holzapfel WH. Improved screening procedure of biogenic amine production by lactic acid bacteria. Int J Food Microbiol. 1999;53:33–41.
Bemmo KUL, Zambou NF, Wang RY, Zhu T, Yin L. Viability and stress response of putative probiotic Lactobacillus plantarum strains in honey environment. Probiotics Antimicrob Prot. 2018;10:629–37.
Kaktcham PM, Temgoua J-B, Zambou FN, Diaz-Ruiz G, Carmen W, María de Lourdes P-C. In Vitro evaluation of the Probiotic and Safety Properties of Bacteriocinogenic and non-bacteriocinogenic lactic acid Bacteria from the Intestines of Nile Tilapia and Common Carp for their use as Probiotics in Aquaculture. Probiotics Antimicro Prot. 2017. https://doi.org/10.1007/s12602-017-9312-8.
AOAC. Association of official analytical chemists, thirteenth ed. In: Official Methods of Analysis. Dr. William Horwitz. Washington. DC. 1980.
Miller GL. Use of Dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31(3):426–8.
AOAC. Official Methods of Analysis, fourteenth ed. Association of Official Analytical Chemists. Arlington. Virginia; 1984.
IUPAC (International Union of Pure and Applied Chemistry). Méthode D’analyse Des matières grasses. Int Dig Health Legis. 1979;46(2):241.
Mensor LL, Menezes FS, Leitao GG, Reis AST. dos, Santos Coube CS, Leitao GS. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res. 2001; 15: 127–130.
Stone H, Sidel JL. Quantitative descriptive analysis: developments, applications, and the future. Food Technol J. 1998;52:48–52.
Boudjelthia KN, Belabbas M, Bekenniche N, Monnoye M, Gérard P, Riazi A. Probiotic Properties of Lactic Acid Bacteria Newly Isolated from Algerian Raw Cow’s Milk. Microorganisms. 2023; 11:2091.
Wang C, Cui Y, Qu X. Mechanisms and improvement of acid resistance in lactic acid bacteria. Arch Microbiol. 2018;200:195–201.
Liu Y, Tang H, Lin Z, Xu P. Mechanisms of acid tolerance in bacteria and prospects in biotechnology and bioremediation. Biotechnol Adv. 2015;33(7):1484–92.
Wu C, Huang J, Zhou R. Progress in engineering acid stress resistance of lactic acid bacteria. Appl Microbiol Biotechnol. 2014;98:1055–63.
Tchamani PL, Kaktcham PM, Foko KEM, Fotso TUD, Ngouénam RJ, Zambou NF. Technological characterisation and probiotic traits of yeasts isolated from Sha’a, a Cameroonian maize-based traditional fermented beverage. Heliyon. 2022;8(10):e1085.
Todorov SD, Holzapfel W, Nero LA. In Vitro evaluation of beneficial properties of bacteriocinogenic Lactobacillus plantarum ST8Sh. Probiotics Antimicrob Proteins. 2016;9:194–203.
Wu WFWJ, Redondo-Solano M, Uribe L, Ching-Jones WR, Usaga J, Barboza N. First characterization of the probiotic potential of lactic acid bacteria isolated from Costa Rican pineapple silages. Peer J. 2021;30:9e12437. https://doi.org/10.7717/peerj.12437.
Junnarkar M, Pawar S, Gaikwad S, Mandal A. Probiotic potential of lactic acid bacteria from fresh vegetables: application in food preservation. Indian J Exp Biol. 2019;57:825–38.
Eke G, Vaysse L, Yao X, Escudero M, Carrière E. Malaquin L. Cell aggregate assembly through microengineering for functional tissue emergence. Cells. 2022; 11(9):1394.
Yadav R, Singh PK, Puniya AK, Shukla P. Catalytic interactions and molecular docking of bile salt hydrolase (BSH) from L. Plantarum RYPR1 and its prebiotic utilization. Front Microbiol. 2017; 7.
Krausova G, Hyrslova I, Hynstova I. Vitro evaluation of Adhesion Capacity. Hydrophobicity, and auto-aggregation of newly isolated potential probiotic strains. Fermentation. 2019;5(4):100.
Beganović J, Kos B, Pavunc AL. Uroić K. Džidara P. Šušković J. Proteolytic activity of probiotic strain Lactobacillus helveticus M92. Anaerobe. 2013;20:58–64.
Lu Y, Tan CW, Chen D, Liu SQ. Potential of three probiotic lactobacilli in transforming star fruit juice into functional beverages. Food Sci Nut. 2018;6(8):2141–50.
Majeed M, Nagabhushanam K, Natarajan S, Sivakumar A, Eshuis-de Ruiter T, Booij-Veurink J, Ali F. Evaluation of genetic and phenotypic consistency of Bacillus coagulans MTCC 5856: a commercial probiotic strain. World J Microbiol Biotechnol. 2016;32:1–12.
Ahmed T, Kanwal R, Ayub N. Influence of temperature on growth pattern of Lactococcus lactis. Streptococcus cremoris and Lactobacillus acidophilus isolated from Camel Milk. Biotechnol. 2006;5:481–8.
Vinderola G. Probiotic bacteria in fermented dairy products. In Anales De La Academia Nacional De Ciencias Exactas. Físicas Y Naturales De Buenos Aires. Anal Acad Nac Cs Ex. 2011;63:61–75.
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Sanders ME. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–14.
Brizuela N, Tymczyszyn EE, Semorile LC, La Hens DV, Delfederico L, Hollmann A, Bravo-Ferrada B. Lactobacillus plantarum as a malolactic starter culture in winemaking: a new (old) player? Electron J Biotechnol. 2019;38:10–8.
Matejčeková Z, Spodniakova S, Dujmić E, Liptakova D, Valík Ľ. Modelling growth of Lactobacillus plantarum as a function of temperature: effects of media. J Food Nut Res. 2019; 58(2).
Ngouénam JR, Kenfack CHM, Kouam EMF, Kaktcham PM, Maharjan R, Ngoufack FZ. Lactic acid production ability of Lactobacillus sp. from four tropical fruits using their by-products as carbon source. Heliyon. 2021;7(5). https://doi.org/10.101.6/j.heliyon.2021.e07079.
Minh NP, Oanh TTK. Fermentation of Ambarella (SPONDIAS DULCIS) wine. Int J Appl Eng Res. 2018;13(2):1324–7.
Filannino P, Cavoski I, Thligene N, Vincentini O, De Angelis M, Silano M, Gobbetti M, Di Cagno R. Correction: lactic acid fermentation of Cactus Cladodes (Opuntia ficus-indica L.) generates flavonoid derivatives with antioxidant and anti-inflammatory properties. PLoS ONE. 2016;11(5):e0155156.
Firdous S, Abdullah N, Ejaz N. Effect of Storage temperature and Time on the vitamin C contents of selected fruits and vegetables: vitamin C in fruits and Vegetable. Biol Sci –PJSIR. 2010;53(4):218–22.
Sangija F, Martin H, Matemu A. Effect of lactic acid fermentation on the nutritional quality and consumer acceptability of African nightshade. Food Sci Nutr. 2022;10(9):3128–42. https://doi.org/10.1002/fsn3.2912.
Lee SK, Kader AA. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol Technol. 2000;20(3):207–20.
Rathod PS, Machewad GM, Deshpande HW. Storage study of prepared probiotic beverage by blending apple and orange juice. J Pharmacogn Phytochem. 2017;6(6):2372–5.
Guanyu J, Guangpeng L, Bin L, Hui T, Ruoyu Z, Xiyun S, Fatao H. Influence on the aroma substances and functional ingredients of apple juice by lactic acid bacteria fermentation. Food Biosci. 2023;51:102337.
Lan T, Lv X, Zhao Q, Lei Y, Gao C, Yuan Q, Sun X, Liu X, Ma T. Optimization of strains for fermentation of kiwifruit juice and effects of mono-and mixed culture fermentation on its sensory and aroma profiles. Food Chem X. 2023;17:100595.
Acknowledgements
The authors express their sincere gratitude to Mr. Tchuenguem Roland. and Mrs. Michelle Tchabou for their participation in data analysis and carrying out the DPPH° scavenging and vitamin C tests.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
In this study, the sensory evaluation involved human subjects. Therefore, these subjects were treated according to the protocol recommended through the Cameroon Bioethics Initiative (Yaoundé-Cameroon) by the ethical clearance number CBI/483/ERCC/CAMBIN.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Foko Kouam, E.M., Tchamani Piame, L., Kouteu, S.S. et al. Probiotic characterisation of Lactiplantibacillus plantarum LO3 and use in the development of a golden apple-based non-dairy probiotic beverage. Syst Microbiol and Biomanuf (2024). https://doi.org/10.1007/s43393-024-00251-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43393-024-00251-1