Abstract
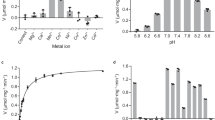
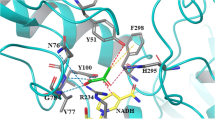
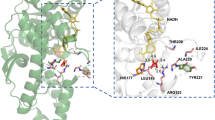
Lactate dehydrogenase and malate dehydrogenase are the two main alpha-hydroxy acid dehydrogenases in the human body. We investigated the catalytic properties of human lactate dehydrogenase LDHC, LDHL6A and malate dehydrogenase MDH1 on aromatic α-keto acids phenylpyruvic acid, p-hydroxyphenylpyruvic acid and 3,4-dihydroxyphenylpyruvic acid. The optimum temperatures for LDHC, LDHL6A, and MDH1 are 37 °C, 35 °C, and 45 °C, respectively; and the optimum pH is 6.5, 6.5, and 5.5, respectively. The Km of LDHC for phenylpyruvic acid and 3,4-dihydroxyphenylpyruvic acid were 0.90 mM and 0.92 mM, respectively. LDHL6A has a high affinity for phenylpyruvic acid and 3,4-dihydroxyphenylpyruvic acid with Km of 0.77 mM and 0.80 mM, respectively; MDH1 has an extremely high affinity (Km = 0.46 mM) and catalytic efficiency (kcat/Km = 23.87 s−1·mM−1) for p-hydroxyphenylpyruvic acid. It also has a high affinity for 3,4-dihydroxyphenylpyruvic acid with a Km of 0.90 mM, but with a low affinity for phenylpyruvic acid (Km = 3.76 mM). The catalytic properties of human LDHC, LDHL6A, and MDH1 for the abovementioned aromatic α-keto acids may be one of the sources of L-phenyllactic acid, L-p-hydroxyphenyllactic acid, and L-3,4-dihydroxyphenyllactic acid in humans.



Similar content being viewed by others
Data availability statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Tang SC, Yang JH. Dual effects of alpha-hydroxy acids on the skin. Molecules. 2018;23(4):863.
Valvona CJ, Fillmore HL, Nunn PB, Pilkington GJ. The regulation and function of lactate dehydrogenase A: therapeutic potential in brain tumor. Brain Pathol. 2016;26(1):3–17.
Goldberg E, Eddy EM, Duan C, Odet F. LDHC: the ultimate testis-specific gene. J Androl. 2010;31(1):86–94.
Wang H, Zhou Z, Lu L, Xu Z, Sha J. Cloning and characterization of a novel intronless lactate dehydrogenase gene in human testis. Int J Mol Med. 2005;15(6):949–53.
Chen X, Gu X, Shan Y, Tang W, Yuan J, Zhong Z, Wang Y, Huang W, Wan B, Yu L. Identification of a novel human lactate dehydrogenase gene LDHAL6A, which activates transcriptional activities of AP1(PMA). Mol Biol Rep. 2009;36(4):669–76.
Burgos C, De Burgos NMG, Coronel CE, Blanco A. Substrate specificity of the lactate dehydrogenase isoenzyme C4 from human spermatozoa and a possible selective assay. J Reprod Fertil. 1979;55(1):101–6.
Mishra D, Banerjee D. Lactate dehydrogenases as metabolic links between tumor and stroma in the tumor microenvironment. Cancers (Basel). 2019;11(6):750.
Gupta GS. LDH-C4: a target with therapeutic potential for cancer and contraception. Mol Cell Biochem. 2012;371(1–2):115–27.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–33.
Lee SM, Dho SH, Ju SK, Maeng JS, Kim JY, Kwon KS. Cytosolic malate dehydrogenase regulates senescence in human fibroblasts. Biogerontology. 2012;13(5):525–36.
Lo AS, Liew CT, Ngai SM, Tsui SK, Fung KP, Lee CY, Waye MM. Developmental regulation and cellular distribution of human cytosolic malate dehydrogenase (MDH1). J Cell Biochem. 2005;94(4):763–73.
Musrati RA, Kollárová M, Mernik N. Malate dehydrogenase: distribution, function and properties. Gen Physiol Biophys. 1998;17(3):193–210.
Friedrichf CA. Biochemical and genetic identity of alpha-keto acid reductase and cytoplasmic malate dehydrogenase from human erythrocytes. Ann Hum Genet. 1988;52:25–37.
Chen YH, Liu SJ, Gao MM, Zeng T, Lin GW, Tan NN, Tang HL, Lu P, Su T, Sun WW, Xie LC, Yi YH, Long YS. MDH2 is an RNA binding protein involved in downregulation of sodium channel Scn1a expression under seizure condition. Biochim Biophys Acta Mol Basis Dis. 2017;1863(6):1492–9.
Kaufman S. A model of human phenylalanine metabolism in normal subjects and in phenylketonuric patients. Proc Natl Acad Sci USA. 1999;96(6):3160–4.
Blau N, van Spronsen FJ, Levy HL. Phenylketonuria. Lancet. 2010;376(9750):1417–27.
Michals K, Lopus M, Matalon R. Phenylalanine metabolites as indicators of dietary compliance in children with phenylketonuria. Biochem Med Metab Biol. 1988;39(1):18–23.
Shen RS. Potent inhibitory effects of tyrosine metabolites on dihydropteridine reductase from human and sheep liver. Biochim Biophys Acta. 1984;785(3):181–5.
Zeng QL, Wang HQ, Liu ZR, Li BG, Zhao YF. Facile synthesis of optically pure (S)-3-p-hydroxyphenyllactic acid derivatives. Amino Acids. 2007;33(3):537–41.
Chernoff HN, Richardson KE. The effect of endogenous l-phenyllactate on oxalate, glycolate, and glyoxylate excretion by phenylketonuric subjects. Clin Chim Acta. 1978;83(1–2):1–6.
Kim JH, Bae KH, Byun JK, Lee S, Kim JG, Lee IK, Jung GS, Lee YM, Park KG. Lactate dehydrogenase-A is indispensable for vascular smooth muscle cell proliferation and migration. Biochem Biophys Res Commun. 2017;492(1):41–7.
Wang T, Yao W, Li J, He Q, Shao Y, Huang F. Acetyl-CoA from inflammation-induced fatty acids oxidation promotes hepatic malate-aspartate shuttle activity and glycolysis. Am J Physiol Endocrinol Metab. 2018;315(4):E496–510.
Pasti AP, Rossi V, Di Stefano G, Brigotti M, Hochkoeppler A. Human lactate dehydrogenase A undergoes allosteric transitions under pH conditions inducing the dissociation of the tetrameric enzyme. 2022. Biosci Rep. https://doi.org/10.1042/BSR20212654.
Acknowledgements
This research did not receive any specific Grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, L., Fan, TP., Wang, M. et al. Human lactate dehydrogenase and malate dehydrogenase possess the catalytic properties to produce aromatic α-hydroxy acid. Syst Microbiol and Biomanuf 3, 627–633 (2023). https://doi.org/10.1007/s43393-022-00154-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43393-022-00154-z