Abstract
Purpose
The aim of the current review is to summarize the current evidence on graft materials used in fusion procedures for spinal deformity corrections.
Methods
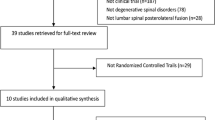
PubMed, Embase, and Cochrane Library were searched for relevant published observational studies and clinical trials using osteobiologics and biomaterials in spinal deformity surgery.
Results
The use of autograft in deformity correction surgeries has been reported in a limited number of studies, with the harvest sites including iliac crest, ribs, and local bone. Various allografts and biologics have been used in the treatment of spinal deformities including idiopathic and degenerative scoliosis, either as stand alone or in combination with autograft. Limited number of studies reported no differences in fusion rates or outcomes. Use of rh-BMP2 in anterior, posterior or front/back approaches showed higher fusion rates than other graft materials in patients with spinal deformities. Due to the limited number of quality studies included in the review, as well as alternative factors, such as costs, availability, and surgeon expertise/preference, no definitive conclusion or recommendations can be made as to the ideal graft choice in spinal deformity surgery.
Conclusions
Most commonly used grafts included autograft, allograft and rh-BMP2, with new biologics and biomaterials constantly emerging in the market. Limited number of high-quality comparative studies and heterogeneity in study design prevented direct comparisons that can lead to meaningful recommendations. Further studies are needed to prove superiority of any single graft material and/or biologic that is also cost-effective and safe.

Similar content being viewed by others
Availability of data and materials
Not applicable.
Code availability
Not applicable.
References
Cahill KS, Chi JH, Day A et al (2009) Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA 302(1):58–66. https://doi.org/10.1001/jama.2009.956
Lipson SJ (2004) Spinal-fusion surgery—advances and concerns. N Engl J Med 350(7):643–644. https://doi.org/10.1056/NEJMp038162
Hofler RC, Swong K, Martin B et al (2018) Risk of pseudoarthrosis after spinal fusion: analysis from the healthcare cost and utilization project. World Neurosurg 120:e194–e202. https://doi.org/10.1016/j.wneu.2018.08.026
Reid JJ, Johnson JS, Wang JC (2011) Challenges to bone formation in spinal fusion. J Biomech 44(2):213–220. https://doi.org/10.1016/j.jbiomech.2010.10.021
Kim YJ, Bridwell KH, Lenke LG et al (2005) Pseudarthrosis in primary fusions for adult idiopathic scoliosis: incidence, risk factors, and outcome analysis. Spine 30(4):468–474. https://doi.org/10.1097/01.brs.0000153392.74639.ea
Wang MC, Chan L, Maiman DJ et al (2007) Complications and mortality associated with cervical spine surgery for degenerative disease in the United States. Spine 32(3):342–347. https://doi.org/10.1097/01.brs.0000254120.25411.ae
Campana V et al (2014) Bone substitutes in orthopaedic surgery: from basic science to clinical practice. J Mater Sci Mater Med 25(10):2445–2461. https://doi.org/10.1007/s10856-014-5240-2
Grabowski G, Cornett CA (2013) Bone graft and bone graft substitutes in spine surgery: current concepts and controversies. J Am Acad Orthop Surg 21(1):51–60. https://doi.org/10.5435/JAAOS-21-01-51
Dimitriou R, Mataliotakis GI, Angoules AG et al (2011) Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury 42(2):S3-15. https://doi.org/10.1016/j.injury.2011.06.015
Duarte RM, Varanda P, Reis RL et al (2017) Biomaterials and bioactive agents in spinal fusion. Tissue Eng Part B Rev 23(6):540–551. https://doi.org/10.1089/ten.TEB.2017.0072
Gupta A, Kukkar N, Sharif K et al (2015) Bone graft substitutes for spine fusion: a brief review. World J Orthop 6(6):449–456. https://doi.org/10.5312/wjo.v6.i6.449
Peterson B, Whang PG, Iglesias R et al (2004) Osteoinductivity of commercially available demineralized bone matrix. Preparations in a spine fusion model. J Bone Joint Surg Am 86(10):2243–2250. https://doi.org/10.2106/00004623-200410000-00016
Tilkeridis K, Touzopoulos P, Ververidis A et al (2014) Use of demineralized bone matrix in spinal fusion,". World J Orthop 5(1):30–37. https://doi.org/10.5312/wjo.v5.i1.30
Aghdasi B, Montgomery SR, Daubs MD et al (2013) A review of demineralized bone matrices for spinal fusion: the evidence for efficacy. Surgeon 11(1):39–48. https://doi.org/10.1016/j.surge.2012.08.001
Mulconrey DS, Bridwell KH, Flynn J, Cronen GA, Rose PS (2008) “Bone morphogenetic protein (RhBMP-2) as a substitute for iliac crest bone graft in multilevel adult spinal deformity surgery: minimum two-year evaluation of fusion,” (in eng). Spine 33(20):2153–2159. https://doi.org/10.1097/BRS.0b013e31817bd91e
Choo QQ, Chiu CK, Lisitha KA et al (2018) Quantitative analysis of local bone graft harvested from the posterior elements during posterior spinal fusion in adolescent idiopathic scoliosis patients. J Orthop 16(1):74–79. https://doi.org/10.1016/j.jor.2018.12.004.PMID:30662243;PMCID:PMC6324765
Franzin FJ, Gotfryd AO, Neto NJ et al (2014) Radiographic and functional evaluation of the iliac bone graft in the treatment of adolescent idiopathic scoliosis. J Pediatr Orthop B 23(4):307–311. https://doi.org/10.1097/BPB.0000000000000037
Kobayashi K, Ando K, Machino M et al (2020) Trends in medical costs for adolescent idiopathic scoliosis surgery in Japan. Global Spine J 10(8):1040–1045. https://doi.org/10.1177/2192568219886265
Milinković ZB, Krneta O, Milicković S et al (2010) Are the additional grafts necessary? Acta Chir Iugosl 57(1):69–72. https://doi.org/10.2298/aci1001069m
Pesenti S, Ghailane S, Varghese JJ et al (2017) Bone substitutes in adolescent idiopathic scoliosis surgery using sublaminar bands: is it useful? A case-control study. Int Orthop 41(10):2083–2090. https://doi.org/10.1007/s00264-017-3512-4
Farshad M, Frey A, Jentzsch T et al (2021) Reducing the kyphosis effect of anterior short thoracolumbar/lumbar scoliosis correction with an autograft fulcrum effect. BMC Musculoskelet Disord 22(1):216. https://doi.org/10.1186/s12891-021-04083-1
Ouellet JA, Johnston CE 2nd (2002) Effect of grafting technique on the maintenance of coronal and sagittal correction in anterior treatment of scoliosis. Spine 27(19):2129–2135. https://doi.org/10.1097/00007632-200210010-00010 (Discussion 2135-6)
Samartzis D, Bow C, Cheung JP et al (2016) Efficacy of postoperative pain management using continuous local anesthetic infusion at the iliac crest bone graft site in patients with adolescent idiopathic scoliosis: a parallel, double-blinded, randomized controlled pilot trial. Global Spine J. 6(3):220–228. https://doi.org/10.1055/s-0035-1558656
Kager AN, Marks M, Bastrom T, Newton PO (2006) Morbidity of iliac crest bone graft harvesting in adolescent deformity surgery. J Pediatr Orthop 26(1):132–134. https://doi.org/10.1097/01.bpo.0000188996.36674.56
Iwai C, Taneichi H, Inami S et al (2013) Clinical outcomes of combined anterior and posterior spinal fusion for dystrophic thoracolumbar spinal deformities of neurofibromatosis-1: fate of nonvascularized anterior fibular strut grafts. Spine 38(1):44–50. https://doi.org/10.1097/BRS.0b013e318261ec74
Johari A, Shingade V, Gajiwala AL et al (2007) The use of irradiated allograft in a paediatric population: an Indian experience. Cell Tissue Bank 8(1):13–22. https://doi.org/10.1007/s10561-006-9001-4
Jones KC, Andrish J, Kuivila T et al (2002) Radiographic outcomes using freeze-dried cancellous allograft bone for posterior spinal fusion in pediatric idiopathic scoliosis. J Pediatr Orthop 22(3):285–289
Watkins RG, Hussain N, Freeman BJ et al (2006) Anterior instrumentation for thoracolumbar adolescent idiopathic scoliosis: do structural interbody grafts preserve sagittal alignment better than morselized rib autografts? Spine 31(20):2337–2342. https://doi.org/10.1097/01.brs.0000240201.14208.68
Izatt MT, Carstens A, Adam CJ et al (2015) Partial intervertebral fusion secures successful outcomes after thoracoscopic anterior scoliosis correction: a low-dose computed tomography study. Spine Deform 3(6):515–527. https://doi.org/10.1016/j.jspd.2015.04.007
Theologis AA, Tabaraee E, Lin T, Spinal Deformity Study Group et al (2015) Type of bone graft or substitute does not affect outcome of spine fusion with instrumentation for adolescent idiopathic scoliosis. Spine 40(17):1345–1351. https://doi.org/10.1097/BRS.0000000000001002
Lowe TG, Alongi PR, Smith DAB et al (2003) Anterior single rod instrumentation for thoracolumbar adolescent idiopathic scoliosis with and without the use of structural interbody support. Spine 28(19):2232–2241. https://doi.org/10.1097/01.BRS.0000085028.70985.39
Hostin R, O’Brien M, McCarthy I et al (2016) Retrospective study of anterior interbody fusion rates and patient outcomes of using mineralized collagen and bone marrow aspirate in multilevel adult spinal deformity surgery. Clin Spine Surg 29(8):E384-388. https://doi.org/10.1097/BSD.0b013e318292468f
Weinzapfel B, Son-Hing JP, Armstrong DG et al (2008) Fusion rates after thoracoscopic release and bone graft substitutes in idiopathic scoliosis. Spine 33(10):1079–1083. https://doi.org/10.1097/BRS.0b013e31816f69b3
Sinagra Z, Cunningham G, Dillon D et al (2020) Proximal junctional kyphosis and rates of fusion following posterior instrumentation and spinal fusion for adolescent idiopathic scoliosis. ANZ J Surg 90(4):597–601. https://doi.org/10.1111/ans.15706
Betz RR, Petrizzo AM, Kerner PJ et al (2006) Allograft versus no graft with a posterior multisegmented hook system for the treatment of idiopathic scoliosis. Spine 31(2):121–127. https://doi.org/10.1097/01.brs.0000194771.49774.77
Knapp DR, Jones ET, Blanco JS et al (2005) Allograft bone in spinal fusion for adolescent idiopathic scoliosis. J Spinal Disord Tech 18:S73-76. https://doi.org/10.1097/01.bsd.0000128694.21405.80
Price CT, Connolly JF, Carantzas AC et al (2003) Comparison of bone grafts for posterior spinal fusion in adolescent idiopathic scoliosis. Spine 28(8):793–798
Wang J, Zhao Y, Shen B et al (2010) Risk factor analysis of proximal junctional kyphosis after posterior fusion in patients with idiopathic scoliosis. Injury 41(4):415–420. https://doi.org/10.1016/j.injury.2010.01.001
Buttermann GR, Glazer PA, Hu SS et al (2001) Anterior and posterior allografts in symptomatic thoracolumbar deformity. J Spinal Disord 14(1):54–66. https://doi.org/10.1097/00002517-200102000-00009
Smith JS, Shaffrey E, Klineberg E et al (2014) Prospective multicenter assessment of risk factors for rod fracture following surgery for adult spinal deformity. J Neurosurg Spine 21(6):994–1003. https://doi.org/10.3171/2014.9.SPINE131176
Bozzio AE, Hu X, Lieberman IH (2019) Cost and clinical outcome of adolescent idiopathic scoliosis surgeries-experience from a nonprofit community hospital. Int J Spine Surg 13(5):474–478. https://doi.org/10.14444/6063
Ong KL, Villarraga ML, Lau E et al (2010) Off-label use of bone morphogenetic proteins in the United States using administrative data. Spine 35:1794–1800. https://doi.org/10.1097/BRS.0b013e3181ecf6e4
Bess S, Line BG, Lafage V et al (2014) Does recombinant human bone morphogenetic protein-2 use in adult spinal deformity increase complications and are complications associated with location of rhBMP-2 use? A prospective, multicenter study of 279 consecutive patients. Spine 39:233–242. https://doi.org/10.1097/BRS.0000000000000104
Luhmann SJ, Bridwell KH, Cheng I et al (2005) Use of bone morphogenetic protein-2 for adult spinal deformity. Spine 30:S110-117. https://doi.org/10.1097/01.brs.0000175184.27407.6a
Ruofeng Y, Cohen JR, Buser Z et al (2016) Trends of posterior long segment fusion with and without recombinant human bone morphogenetic protein 2 in patients with scoliosis. Global Spine J 6:422–431. https://doi.org/10.1055/s-0035-1564416
Mulconrey DS, Bridwell KH, Flynn J et al (2008) Bone morphogenetic protein (RhBMP-2) as a substitute for iliac crest bone graft in multilevel adult spinal deformity surgery: minimum two-year evaluation of fusion. Spine 33:2153–2159. https://doi.org/10.1097/BRS.0b013e31817bd91e
Maeda T, Buchowski JM, Kim YJ et al (2009) Long adult spinal deformity fusion to the sacrum using rhBMP-2 versus autogenous iliac crest bone graft. Spine 34:2205–2212. https://doi.org/10.1097/BRS.0b013e3181b0485c
Kim HJ, Buchowski JM, Zebala LP et al (2013) RhBMP-2 is superior to iliac crest bone graft for long fusions to the sacrum in adult spinal deformity: 4- to 14-year follow-up. Spine 38:1209–1215. https://doi.org/10.1097/BRS.0b013e31828b656d
Puvanesarajah V, Jain A, Cancienne JM et al (2017) BMP use and the risk of revision surgery after long posterolateral fusions in the elderly. Clin Spine Surg 30:E931–E937. https://doi.org/10.1097/BSD.0000000000000489
Safaee MM, Dalle Ore CL, Zygourakis CC et al (2019) Estimating a price point for cost-benefit of bone morphogenetic protein in pseudarthrosis prevention for adult spinal deformity surgery. J Neurosurg Spine. https://doi.org/10.3171/2018.12.SPINE18613
Jain A, Yeramaneni S, Kebaish KM et al (2020) Cost-utility analysis of rhBMP-2 use in adult spinal deformity surgery. Spine 45:1009–1015. https://doi.org/10.1097/BRS.0000000000003442
Yoo JS, Ahn J, Patel DS et al (2019) An evaluation of biomaterials and osteobiologics for arthrodesis achievement in spine surgery. Ann Transl Med 7:S168. https://doi.org/10.21037/atm.2019.06.80
Ameri E, Behtash H, Mobini B et al. Bioactive glass versus autogenous iliac crest bone graft in adolescent idiopathic scoliosis surgery. Acta Medica Iranica 2009: 41–45
Hing KA, Revell PA, Smith N et al (2006) Effect of silicon level on rate, quality and progression of bone healing within silicate-substituted porous hydroxyapatite scaffolds. Biomaterials 27:5014–5026. https://doi.org/10.1016/j.biomaterials.2006.05.039
Harshavardhana NS, Noordeen MH (2015) Surgical results with the use of silicated calcium phosphate (SiCaP) as bone graft substitute in posterior spinal fusion (PSF) for adolescent idiopathic scoliosis (AIS). Scoliosis 10:27. https://doi.org/10.1186/s13013-015-0051-x
Mashoof AA, Siddiqui SA, Otero M et al (2002) Supplementation of autogenous bone graft with coralline hydroxyapatite in posterior spine fusion for idiopathic adolescent scoliosis. Orthopedics 25:1073–1076
Muschik M, Ludwig R, Halbhubner S et al (2001) Beta-tricalcium phosphate as a bone substitute for dorsal spinal fusion in adolescent idiopathic scoliosis: preliminary results of a prospective clinical study. Eur Spine J 10(2):S178-184. https://doi.org/10.1007/s005860100271
Delecrin J, Takahashi S, Gouin F et al (2000) A synthetic porous ceramic as a bone graft substitute in the surgical management of scoliosis: a prospective, randomized study. Spine 25(5):563-9. https://doi.org/10.1097/00007632-200003010-00006
Ilharreborde B, Morel E, Fitoussi F et al (2008) Bioactive glass as a bone substitute for spinal fusion in adolescent idiopathic scoliosis: a comparative study with iliac crest autograft. J Pediatr Orthop 28:347–351. https://doi.org/10.1097/BPO.0b013e318168d1d4
Lerner T, Bullmann V, Schulte TL et al (2009) A level-1 pilot study to evaluate of ultraporous beta-tricalcium phosphate as a graft extender in the posterior correction of adolescent idiopathic scoliosis. Eur Spine J 18:170–179. https://doi.org/10.1007/s00586-008-0844-1
Lerner T, Liljenqvist U (2013) Silicate-substituted calcium phosphate as a bone graft substitute in surgery for adolescent idiopathic scoliosis. Eur Spine J 22(2):S185-194. https://doi.org/10.1007/s00586-012-2485-7
Bazylinska U, Lewinska A, Lamch L, Wilk KA (2014) Polymeric nanocapsules and nanospheres for encapsulation and long sustained release of hydrophobic cyanine-type photosensitizer. Colloids Surf A Physicochem Eng Asp 442:42–49. https://doi.org/10.1016/j.colsurfa.2013.02.023
Viswanathan VK, Rajaram Manoharan SR, Subramanian S et al (2019) Nanotechnology in spine surgery: a current update and critical review of the literature. World Neurosurg 123:142–155. https://doi.org/10.1016/j.wneu.2018.11.035
Lee SS, Hsu EL, Mendoza M et al (2015) Gel scaffolds of BMP-2-binding peptide amphiphile nanofibers for spinal arthrodesis. Adv Healthc Mater 4(1):131–141. https://doi.org/10.1002/adhm.201400129
Stylios G, Wan T, Giannoudis P (2007) Present status and future potential of enhancing bone healing using nanotechnology. Injury 38(1):S63-74. https://doi.org/10.1016/j.injury.2007.02.011
Brannigan K, Griffin M (2016) An update into the application of nanotechnology in bone healing. Open Orthop J 30(10):808–823. https://doi.org/10.2174/1874325001610010808
Funding
No funding was received for this review paper.
Author information
Authors and Affiliations
Contributions
KEC: contribution to the study design, drafted a section of the manuscript, approved final version, agree to be accountable for all aspects of the work. MKM: contribution to acquisition and analysis, drafted a section of the manuscript, approved final version, agree to be accountable for all aspects of the work. ZF: contribution to acquisition and analysis, drafted a section of the manuscript, approved final version, agree to be accountable for all aspects of the work. ES: contribution to acquisition and analysis, drafted a section of the manuscript, approved final version, agree to be accountable for all aspects of the work. ZB: contribution to study design, acquisition, drafted and critically revised the manuscript, approved final version, agree to be accountable for all aspects of the work. JCW: contribution to study design, critically revised the manuscript, approved final version, agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflicts of interest
There are no conflicts of interest in this study. Disclosures outside of submitted work: ZB-consultancy: Cerapedics (past), Xenco Medical (past), AO Spine (past); Research Support: SeaSpine (past, paid to the institution), Medical Metrics (past, paid directly to institution), Next Science (past, paid directly to institution); North American Spine Society: committee member; Lumbar Spine Society: Co-chair Education committee, AOSpine Knowledge Forum Degenerative: Associate member; AOSNA Research committee—committee member; JCW—Royalties—Biomet, Seaspine, Synthes; Investments/Options—Bone Biologics, Pearldiver, Electrocore, Surgitech; Board of Directors—AO Foundation, American Orthopaedic Association; Fellowship Funding (paid to institution): AO Foundation.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chang, KE., Mesregah, M.K., Fresquez, Z. et al. Use of graft materials and biologics in spine deformity surgery: a state-of-the-art review. Spine Deform 10, 1217–1231 (2022). https://doi.org/10.1007/s43390-022-00529-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43390-022-00529-1