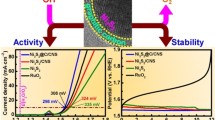
Abstract
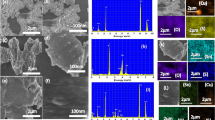
To drive clean and sustainable fuel production via water electrolysis, development of high-performing, cost-effective electrocatalysts rich in earth elements without relying on precious metals or costly materials is crucial. In this study, strontium selenide (SrSe), copper sulfide (CuS), and composite SrSe@CuS via a traditional coprecipitate method under alkaline conditions are synthesized. Characterization techniques including X-ray diffraction, Transmission electron microscopy, Field emission scanning electron microscopy, and Brunauer–Emmett–Teller surface area analysis are employed to analyze the structure, morphology, and surface characteristics. The larger surface area of 123 m2 g−1 and lower crystalline size (46.43 nm) of SrSe@CuS nanosheets show more active sites for oxygen evolution reaction. The oxygen evolution activity displayed overpotentials of 290 mV, a lower tafel slope of 67 mV dec−1, and Lower charge transfer resistance (RCT) values of SrSe@CuS nanosheets (1.82 Ω) surpassing the individual SrSe and CuS nanosheets. Notably, the SrSe@CuS nanosheets exhibited remarkable stability, maintaining an oxygen evolution reaction (OER) activity of 10 mA cm−2 for over 50 h and sustaining a negligible loss in performance even after 50,000 cycles of repetitive cyclivoltammetry scans. These findings highlight their potential applicability in energy conversion and storage systems.
Graphical abstract









Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
A.W. Rennuit-Mortensen, K.D. Rasmussen, M. Grahn, How replacing fossil fuels with electrofuels could influence the demand for renewable energy and land area. Smart Energy 10, 100107 (2023)
A. Zahoor et al., The carbon neutrality feasibility of worldwide and in China’s transportation sector by E-car and renewable energy sources before 2060. J. Energy Storage 61, 106696 (2023)
F.Z. Ainou, M. Ali, M. Sadiq, Green energy security assessment in Morocco: green finance as a step toward sustainable energy transition. Environ. Sci. Pollut. Res. 30(22), 61411–61429 (2023)
W. Azam, I. Khan, S.A. Ali, Alternative energy and natural resources in determining environmental sustainability: a look at the role of government final consumption expenditures in France. Environ. Sci. Pollut. Res. 30(1), 1949–1965 (2023)
S.S. Ali et al., Bioplastic production in terms of life cycle assessment: a state-of-the-art review. Environ. Sci. Ecotechnol. 15, 100254 (2023)
M.M. Rahman et al., Powering agriculture: present status, future potential, and challenges of renewable energy applications. Renew. Energy 188, 731–749 (2022)
A. Rahman, O. Farrok, M.M. Haque, Environmental impact of renewable energy source based electrical power plants: solar, wind, hydroelectric, biomass, geothermal, tidal, ocean, and osmotic. Renew. Sustain. Energy Rev. 161, 112279 (2022)
B. Roose et al., Local manufacturing of perovskite solar cells, a game-changer for low-and lower-middle income countries? Energy Environ. Sci. 15(9), 3571–3582 (2022)
U.S. Meda, Y.V. Rajyaguru, A. Pandey, Generation of green hydrogen using self-sustained regenerative fuel cells: opportunities and challenges. Int. J. Hydrogen Energy 48, 28289–28314 (2023)
V.M. Avargani et al., A comprehensive review on hydrogen production and utilization in North America: prospects and challenges. Energy Convers. Manage. 269, 115927 (2022)
M. Gopinath, R. Marimuthu, A review on solar energy-based indirect water-splitting methods for hydrogen generation. Int. J. Hydrogen Energy 47(89), 37742–37759 (2022)
P. Pattanayak et al., Recent progress in perovskite transition metal oxide-based photocatalyst and photoelectrode materials for solar-driven water splitting. J. Environ. Chem. Eng. 10, 108429 (2022)
A.R. Fareza et al., Nanoscale metal oxides–2D materials heterostructures for photoelectrochemical water splitting—a review. J. Mater. Chem. A 10(16), 8656–8686 (2022)
M. Nasser, H. Hassan, Assessment of hydrogen production from waste heat using hybrid systems of Rankine cycle with proton exchange membrane/solid oxide electrolyzer. Int. J. Hydrogen Energy 48(20), 7135–7153 (2023)
Y. Wu et al., Recent progress in biomass-derived nanoelectrocatalysts for the sustainable energy development. Fuel 323, 124349 (2022)
A.P. Demchenko, Proton transfer reactions: from photochemistry to biochemistry and bioenergetics. BBA Adv. 3, 100085 (2023)
H. Jing et al., Theory-oriented screening and discovery of advanced energy transformation materials in electrocatalysis. Adv. Powder Mater. 1(1), 100013 (2022)
N.A. Kamaruzaman et al., Recent advances in transition metals-based materials as electrocatalysts for water splitting. Int. J. Electrochem. Sci. 18, 100187 (2023)
M. Ďurovič, J. Hnát, K. Bouzek, Electrocatalysts for the hydrogen evolution reaction in alkaline and neutral media. A comparative review. J. Power. Sources 493, 229708 (2021)
E. Loni, A. Shokuhfar, M. Siadati, Cobalt-based electrocatalysts for water splitting: an overview. Catal. Surv. Asia 25, 114–147 (2021)
X. Zhang, L. Wang, H. Fu, Recent advances in rechargeable Zn-based batteries. J. Power. Sources 493, 229677 (2021)
M.U. Nisa et al., CdSe supported SnO2 nanocomposite with strongly hydrophilic surface for enhanced overall water splitting. Fuel 321, 124086 (2022)
A.G. Abid et al., Stainless steel supported NiS/CeS nanocomposite for significantly enhanced oxygen evolution reaction in alkaline media. J. Solid State Electrochem. 26(10), 2107–2118 (2022)
Z. Chen et al., Controllable design of nanoworm-like nickel sulfides for efficient electrochemical water splitting in alkaline media. Mater. Today Energy 18, 100573 (2020)
A. Farhan et al., Transition-metal sulfides with excellent hydrogen and oxygen reactions: a mini-review. J. Solid State Chem. 329, 124445 (2023)
S.K. Ramesh, V. Ganesan, J. Kim, FeSe2-CoSe2/CoSe2 yolk-shell nanoboxes as superior electrocatalysts for the oxygen evolution reaction. Mater. Lett. 323, 132573 (2022)
K. Wang et al., Selenide/sulfide heterostructured NiCo2Se4/NiCoS4 for oxygen evolution reaction, hydrogen evolution reaction, water splitting and Zn-air batteries. Electrochim. Acta 368, 137584 (2021)
J. Yang et al., Metal-organic framework-derived FeS2/CoNiSe2 heterostructure nanosheets for highly-efficient oxygen evolution reaction. Appl. Surf. Sci. 578, 152016 (2022)
A. Raveendran, M. Chandran, R. Dhanusuraman, A comprehensive review on the electrochemical parameters and recent material development of electrochemical water splitting electrocatalysts. RSC Adv. 13(6), 3843–3876 (2023)
J. Li et al., Optimizing hydrogen production by alkaline water decomposition with transition metal-based electrocatalysts. Environ. Chem. Lett. 31, 2583–2617 (2023)
X. Guan et al., Probing the national development from heavy metals contamination in river sediments. J. Clean. Prod. 419, 138164 (2023)
S. Vignesh, H. Kim, Influence of molybdenum on Co3O4 coupled N-doped reduced graphene oxide composite for improved electrocatalytic alkaline oxygen evolution reaction: stability and mechanism insights. Int. J. Hydrogen Energy 48, 37234–37247 (2023)
X. Yang et al., Heterogeneous ultra-thin FeCo-LDH@ Co (OH) 2 nanosheets facilitated electrons transfer for oxygen evolution reaction. Chem. Eng. J. 472, 145076 (2023)
G. Calzaferri et al., Multiple equilibria description of type H1 hysteresis in gas sorption isotherms of mesoporous materials. Mater. Chem. Phys. 296, 127121 (2023)
Y. Wang et al., Investigation of photo (electro) catalytic water splitting to evolve H2 on Pt-g-C3N4 nanosheets. Int. J. Hydrogen Energy 47(65), 28007–28018 (2022)
J. Jiang et al., Superwetting molybdenum-based sulfide/phosphide heterostructures for efficient water electrolysis and solar thermoelectricity self-powered hydrogen production. Appl. Surf. Sci. 631, 157482 (2023)
H. Tong, Y. Jiang, L. Xia, Enhancing photoelectrochemical water oxidation activity of BiVO4 photoanode through the Co-catalytic effect of Ni (OH) 2 and carbon quantum dots. Int. J. Hydrogen Energy 48, 36694–36706 (2023)
M. Liu et al., Construction of an electrode with hierarchical three-dimensional NiFe-oxyhydroxides by two-step electrodeposition for large-current oxygen evolution reaction. Int. J. Hydrogen Energy 51, 626–637 (2023)
S. Bi et al., (Digital Presentation) The effects of sulfur-oxygen ratio at the heterogeneous interface on oxygen evolution reaction performance of Ni3S2@ Ni3Fe catalysts for alkaline water electrolysis. ECS Trans. 111(4), 97 (2023)
Y. Sun et al., Deep neural network based battery impedance spectrum prediction using only impedance at characteristic frequencies. J. Power. Sources 580, 233414 (2023)
S. Feng, et al., Self-assembled heterojunction CoSe2@ CoO catalysts for efficient seawater electrolysis. Electrochim. Acta 463, 142870 (2023)
Y. Li et al., Effect of molybdenum phosphorus-based single/double-atom catalysts on hydrogen evolution reaction: first principles. Int. J. Hydrogen Energy 51, 957–969 (2023)
N. Chen et al., Facile fabrication of flower-like CuS/MnCO3 microspheres clusters on nickel foam as an efficient bifunctional catalyst for overall water splitting. Int. J. Hydrogen Energy 46(38), 19948–19961 (2021)
W. Li et al., Heterostructured CoSe2/FeSe2 nanoparticles with abundant vacancies and strong electronic coupling supported on carbon nanorods for oxygen evolution electrocatalysis. ACS Sustain. Chem. Eng. 8(11), 4658–4666 (2020)
J.A. Rajesh et al., Bifunctional NiCo2Se4 and CoNi2Se4 nanostructures: efficient electrodes for battery-type supercapacitors and electrocatalysts for the oxygen evolution reaction. J. Ind. Eng. Chem. 79, 370–382 (2019)
Y. Yang et al., An interfacial electron transfer on tetrahedral NiS2/NiSe2 heterocages with dual-phase synergy for efficiently triggering the oxygen evolution reaction. Small 16(1), 1905083 (2020)
S. Ni et al., Interfacial engineering of the NiSe2/FeSe2 pp heterojunction for promoting oxygen evolution reaction and electrocatalytic urea oxidation. Appl. Catal. B 299, 120638 (2021)
O.A. Oyetade, R.J. Kriek, NiSe-Ni3Se2/multiwalled carbon nanotube composites as efficient electrocatalysts for the oxygen evolution reaction in alkaline media. Electrocatalysis 11, 35–45 (2020)
R. Bose et al., Self-supportive bimetallic selenide heteronanostructures as high-efficiency electro (pre) catalysts for water oxidation. ACS Sustain. Chem. Eng. 9(38), 13114–13123 (2021)
X. Zhao et al., Electrical and structural engineering of cobalt selenide nanosheets by Mn modulation for efficient oxygen evolution. Appl. Catal. B 236, 569–575 (2018)
G. Zhu et al., Nanocomposites based on CoSe2-decorated FeSe2 nanoparticles supported on reduced graphene oxide as high-performance electrocatalysts toward oxygen evolution reaction. ACS Appl. Mater. Interfaces 10(22), 19258–19270 (2018)
S. Sadiq et al., Synergistic modification of end groups in Quinoxaline fused core-based acceptor molecule to enhance its photovoltaic characteristics for superior organic solar cells. J. Mol. Graph. Model. 123, 108518 (2023)
Acknowledgements
This work was funded by the Researchers Support Project Number (RSP2024R265), King Saud University, Riyadh, Saudi Arabia
Author information
Authors and Affiliations
Contributions
All have done equal contribution.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Junaid, A., Abdullah, M., Bano, N. et al. Facile synthesis of strontium selenide supported copper sulfide hybrid nanosheets as an efficient electrode for high-performance OER. J. Korean Ceram. Soc. 61, 469–481 (2024). https://doi.org/10.1007/s43207-024-00372-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43207-024-00372-2