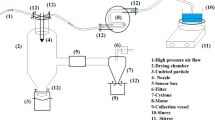
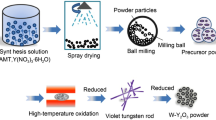
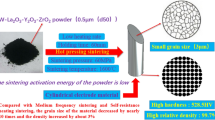
Abstract
Yttria (Y2O3) is a representative material having excellent plasma resistance, and yttria powder, applied to semiconductor components and thermal spray coating, requires excellent sinterability and flowability, for which particle shape and size are important factors. In the present study, to synthesize yttria powder having various shape applicable to many industrial areas, the morphology of yttria particles was controlled through hydro(solvo)thermal synthesis. The yttria powder was synthesized using deionized water, ethylene glycol and glycerol as solvents. The precursor concentrations and the synthesis conditions such as synthesis temperature and time were examined. The particle shape and size of the yttria powder were adjusted to plate-type, rod-type and spherical-type depending on the applied solvent, precipitant and synthesis temperature. The thermal treatment following the hydro(solvo)thermal synthesis did not have a significant effect on the shape of the yttria particles. Cubic single-phase yttria was observed at a calcination temperature of 450 °C or higher, and the crystal phase further developed as the thermal treatment temperature increased. In the powder synthesized using deionized water, the crystal phase developed mostly on the [222] direction depending on the temperature during oriented attachment. On the other hand, in the powder synthesized using ethylene glycol and glycerol as a solvent, the crystal phase developed homogeneously in all crystalline directions.













Similar content being viewed by others
References
J.H. Pee, J.C. Park, K.T. Hwang, S.R. Kim, W.S. Cho, Res. Chem. Intermed. 36, 801 (2010). https://doi.org/10.1007/s11164-010-0184-8
J.H. Lee, S.H. Bea, Korean. J. Mater. Res. 27(8), 445 (2017). https://doi.org/10.3740/MRSK.2017.27.8.445
H.W. Lee, H.I. JI, J.H. Lee, B.K. Kim, K.J. Yoon, J.W. Son, J. Korean Ceram. Soc. 56(2), 130 (2019). https://doi.org/10.4191/kcers.2019.56.2.04
S.K. Rha, M.J. Lee, Y.S. Lee, J. Korean Ceram. Soc. 57, 338 (2020). https://doi.org/10.1007/s43207-020-00034-z
W.K. Jung, H.J. Ma, S.W. Jung, D.K. Kim, J. Am. Ceram. Soc. 100, 1876 (2017). https://doi.org/10.1111/jace.14776
W. Liu, L. Jin, S. Wang, Mater. Chem. Phys. 232, 471 (2019). https://doi.org/10.1016/j.matchemphys.2019.05.018
X. Wang, Y. Hu, X. Meng, Y. Li, M. Zhu, H. Jin, J. Rare Earths 33(7), 706 (2015). https://doi.org/10.1016/S1002-0721(14)60474-9
B.V. Hao, P.T. Huy, T.N. Khiem, N.T.T. Ngan, P.H. Duong, J. Phys. Conf. Ser. 187, 012074 (2009). https://doi.org/10.1088/1742-6596/187/1/012074
D. Nunes, A. Pimentel, L. Santos, P. Barquinha, L. Pereira, E. Fortunato, R. Martins, Metal oxide nanostructures synthesis, properties and applications (Elsevier, Chisinau, 2019), pp. 37–39
M.G. Siahroudi, A.A. Daryakenari, Y.B. Molamahaleh, Q. Cao, M.A. Darayakenari, J.J. Delaunay, H. Siavoshi, F. Molaei, Int. J. Hydrogen Energy 45(55), 30357 (2020). https://doi.org/10.1016/j.ijhydene.2020.08.007
V. Maheskumar, T. Selvaraju, B. Vidhya, Int. J. Hydrogen Energy 43(51), 22861 (2018). https://doi.org/10.1016/j.ijhydene.2018.09.072
X. Li, Q. Li, J. Wang, J. Li, J. Lumin. 124(2), 351 (2007). https://doi.org/10.1016/j.jlumin.2006.04.007
Towata, M. Sivakumar, K. Yasui, T. Tuziuti, T. Kozuka, Y. Iida (2008) J. Mater. Sci. 43:1214. https://doi.org/10.1007/s10853-007-2287-1
Q. Tang, Z. Liu, S. Li, S. Zhanga, X. Liu, Y. Qian, J. Cryst. Growth 259(1–2), 208 (2003). https://doi.org/10.1016/S0022-0248(03)01590-2
Z. Liu, B. Wu, D. Xiang, Y. Zhu, Mater. Res. Bull. 47(11), 3753 (2012). https://doi.org/10.1016/j.materresbull.2012.06.026
M.W. Shafer, R. Roy, J. Am. Ceram. Soc. 42(11), 563 (1959). https://doi.org/10.1111/j.1151-2916.1959.tb13574.x
H. Xian, J. Zhu, H. Tang, X. Liang, H. He, Y. Xi, CrystEngComm 18, 8823 (2016). https://doi.org/10.1039/C6CE01692H
T. Liu, Z. Jin, J. Li, J. Wang, D. Wang, J. Lai, H. Du, CrystEngComm 15, 8903 (2013). https://doi.org/10.1039/C3CE41500G
R.L. Penn, J.F. Banfield, Am. Assoc. Adv. Sci. 281(5379), 969 (1998). https://doi.org/10.1126/science.281.5379.969
R.L. Penn, J.F. Banfield, Am. Mineral. 83(9–10), 1077 (1998). https://doi.org/10.2138/am-1998-9-1016
R.L. Penn, J.F. Banfield, Geochim. Cosmochim. Acta 63, 1549 (1999). https://doi.org/10.1016/S0016-7037(99)00037-X
J.A. Soltis, R.L. Penn, in Iron Oxides: From Nature to Applications, ed. By D. Faivre, R. B. Frankel (Wiley, New York, 2016), pp. 243–268. https://doi.org/10.1002/9783527691395.ch11
W. He, K. Wen, Y. Niu, Nanocrystals from Oriented-Attachment for Energy Applications, (Springer, New York, 2018), pp. 1–13. https://doi.org/10.1007/978-3-319-72432-4
X. Peng, L. Manna, W. Yang, J. Wickham, E. Scher, A. Kadavanich, A.P. Alivisatos, Nature 404, 59 (2000). https://doi.org/10.1038/35003535
A.L. Patterson, Phys. Rev. 56, 978 (1939). https://doi.org/10.1103/PhysRev.56.978
K. Nakashima, Y. Toshima, Y. Kobayashi, Y. Ishikawa, M. Kakihana, J. Asian. Ceram. Soc. 7(4), 544 (2019). https://doi.org/10.1080/21870764.2019.1683955
J. Wu, X. Lü, L. Zhang, F. Huang, F. Xu, Eur. J. Inorg. Chem. 2009(19), 2789 (2009). https://doi.org/10.1002/ejic.200900199
Acknowledgements
This Research was supported by Research Funds of Mokpo National University in 2021.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oh, BH., Lee, SJ. Control of particle morphology and size of yttria powder prepared by hydro(solvo)thermal synthesis. J. Korean Ceram. Soc. 59, 436–443 (2022). https://doi.org/10.1007/s43207-022-00186-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43207-022-00186-0