Abstract
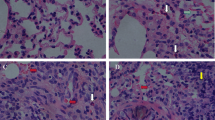
Several studies have pointed to fine particulate matter (PM2.5) as the main responsible for air pollution toxic effects. Indeed, PM2.5 may not only cause respiratory and cardiovascular abnormalities but it may also affect other organs such as the liver. Be that as it may, only a few studies have evaluated the PM2.5 effects on hepatic tissue. Moreover, most of them have not analyzed the relationship between particles composition and toxicological effects. In this study, healthy rats were subjected to urban levels of PM2.5 particles in order to assess their structural and functional effects on the liver. During the exposure periods, mean PM2.5 concentrations were slightly higher than the value suggested by the daily guideline of the World Health Organization. The exposed rats showed a hepatic increase of Cr, Zn, Fe, Ba, Tl and Pb levels. This group also showed leukocyte infiltration, sinusoidal dilation, hydropic inclusions and alterations in carbohydrates distribution. These histologic lesions were accompanied by serological changes, such as increase of total cholesterol and triglycerides, as well as genotoxic damage in their nuclei. We also observed significant associations between several biomarkers and PM2.5 composition. Our results show that exposure to low levels of PM2.5 might cause histologic and serological changes in liver tissue, suggesting that PM2.5 toxicity is influenced not only by their concentration but also by their composition and the exposure frequency.



Similar content being viewed by others
Abbreviations
- PM2.5 :
-
Fine particulate matter
- WHO:
-
World Health Organization
- PAHs:
-
Polycyclic aromatic hydrocarbons
- HI:
-
Harvard Impactor
- PTFE:
-
Polytetrafluoroethylene
- Nap:
-
Naphthalene
- Acy:
-
Acenaphthylene
- Acp:
-
Acenaphthene
- Flr:
-
Fluorene
- Phe:
-
Phenanthrene
- Ant:
-
Anthracene
- Flt:
-
Fluoranthene
- Pyr:
-
Pyrene
- B[a]A:
-
Benzo[a]anthracene
- Chr:
-
Chrysene
- B[b]F:
-
Benzo[b]fluoranthene
- B[k]F:
-
Benzo[k]fluoranthene
- B[a]P:
-
Benzo[a]pyrene
- D[ah]A:
-
Dibenzo[a,h]anthracene
- Ind:
-
Indeno[1,2,3-c,d]pyrene
- B[ghi]P:
-
Benzo[g,h,i]perylene
- PBS:
-
Phosphate buffered saline solution
- HBSS:
-
Hank’s balanced saline solution
- HDL:
-
High density lipoproteins
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- HE:
-
Hematoxylin/eosin stain
- PAS:
-
Periodic acid-Schiff stain
- TMV:
-
Tail moment value
- TIM:
-
Total inhaled mass
- EF:
-
Enrichment factor
References
World Health Organization (2016) Ambient air pollution: a global assessment of exposure and burden of disease. World Health Organization, Geneva
Riva D, Magalhaes CB, Lopes A, Lancas T, Mauad T, Malm O et al (2011) Low dose of fine particulate matter (PM2.5) can induce acute oxidative stress, inflammation and pulmonary impairment in healthy mice. Inhal Toxicol 23:257–267
Wang C, Tu Y, Yu Z, Lu R (2015) PM2.5 and cardiovascular diseases in the elderly: an overview. Int J Environ Res Public Health 12:8187–8197
Kioumourtzoglou MA, Schwartz JD, Weisskopf MG, Melly SJ, Wang Y, Dominici F, Zanobetti A (2015) Long-term PM2.5 exposure and neurological hospital admissions in the northeastern United States. Environ Health Perspect 124:23–29
Zheng Z, Zhang X, Wang J, Dandekar A, Kim H, Qiu Y et al (2015) Exposure to fine airborne particulate matters induces hepatic fibrosis in murine models. J Hepatol 63:1397–1404
Miller MR, Raftis JB, Langrish JP, McLean SG, Samutrtai P, Connell SP et al (2017) Inhaled nanoparticles accumulate at sites of vascular disease. ACS Nano 11:4542–4552
Bourdon JA, Saber AT, Jacobsen NR, Jensen KA, Madsen AM, Lamson JS et al (2012) Carbon black nanoparticle instillation induces sustained inflammation and genotoxicity in mouse lung and liver. Part Fibre Toxicol 9:5
Ge CX, Qin YT, Lou DS, Li Q, Li YY, Wang ZM et al (2017) iRhom2 deficiency relieves TNF-α associated hepatic dyslipidemia in long-term PM2.5-exposed mice. Biochem Biophys Res Commun 493:1402–1409
Umezawa M, Nakamura M, El-Ghoneimy AA, Onoda A, Shaheen HM, Hori H et al (2018) Impact of diesel exhaust exposure on the liver of mice fed on omega-3 polyunsaturated fatty acids-deficient diet. Food Chem Toxicol 111:284–294
Zheng Z, Xu X, Zhang X, Wang A, Zhang C, Hüttemann M et al (2013) Exposure to ambient particulate matter induces a NASH-like phenotype and impairs hepatic glucose metabolism in an animal model. J Hepatol 58:148–154
Jackson P, Hougaard KS, Boisen AMZ, Jacobsen NR, Jensen KA, Møller P et al (2012) Pulmonary exposure to carbon black by inhalation or instillation in pregnant mice: effects on liver DNA strand breaks in dams and offspring. Nanotoxicology 6:486–500
Agency for Toxic Substances and Disease Registry (1995) Toxicological profile for polycyclic aromatic hydrocarbons. U.S. Department od Health and Human Services. Public Health Service
Busso IT, Vera A, Mateos AC, Amarillo AC, Carreras H (2017) Histological changes in lung tissues related with sub-chronic exposure to ambient urban levels of PM2.5 in Córdoba, Argentina. Atmos Environ 167:616–624
Barile FA (2013) Principles of toxicology testing. CRC Press, Boca Raton
United States Environmental Protection Agency (2017) Health effects. Notebook for hazardous air pollutants
Tames MF, Tavera Busso IY, Carreras HA (2019) Optimización de método para la determinación de hidrocarburos aromáticos policíclicos asociados a material particulado. Rev Int Contam Ambie 35:387–395
Wise SA, Sander LC, May WE (1993) Determination of polycyclic aromatic hydrocarbons by liquid chromatography. J Chromatogr A 642(1–2):329–349
Burtis CA, Bruns DE (2014) Tietz fundamentals of clinical chemistry and molecular diagnostics-e-book. Elsevier Health Sciences, Amsterdam
Bancroft JD, Floyd AD, Suvarna SK (2013) Bancroft’s theory and practice of histological techniques. Elsevier, Amsterdam
Busso IT, Mateos AC, Juncos LI, Canals N, Carreras HA (2018) Kidney damage induced by sub-chronic fine particulate matter exposure. Environ Int 121:635–642
Kumar V, Abbas AK, Aster JC (2017) Robbin’s basic pathology e-book. Elsevier Health Sciences, Amsterdam
Kabata-Pendias A, Mukherjee AB (2007) Trace elements from soil to human. Springer, Berlin
Slezakova K, Castro D, Delerue-Matos C, da Conceição Alvim-Ferraz M, Morais S, do Carmo Pereira M (2013) Impact of vehicular traffic emissions on particulate-bound PAHs: levels and associated health risks. Atmos Res 127:141–147
Achad M, López ML, Ceppi S, Palancar GG, Tirao G, Toselli BM (2014) Assessment of fine and sub-micrometer aerosols at an urban environment of Argentina. Atmos Environ 92:522–532
Tsapakis M, Stephanou EG (2005) Occurrence of gaseous and particulate polycyclic aromatic hydrocarbons in the urban atmosphere: study of sources and ambient temperature effect on the gas/particle concentration and distribution. Environ Pollut 133:147–156
Wellen KE, Hotamisligil GS (2005) Inflammation, stress, and diabetes. J Clin Invest 115:1111–1119
Olcese LE, Toselli BM (2002) Some aspects of air pollution in Córdoba, Argentina. Atmos Environ 36:299–306
Bermudez GM, Jasan R, Plá R, Pignata ML (2012) Heavy metals and trace elements in atmospheric fall-out: their relationship with topsoil and wheat element composition. J Hazard Mater 213:447–456
Ferreyroa GV, Montenegro AC, Tudino MB, Lavado RS, Molina FV (2014) Time evolution of Pb(II) speciation in Pampa soil fractions. Chem Spec Bioavailab 26:210–218
Litter MI, Nicolli HB, Meichtry M, Quici N, Bundschuh J, Bhattacharya P, Naidu R (eds) (2014) One century of the discovery of arsenicosis in Latin America (1914–2014) As2014: proceedings of the 5th international congress on arsenic in the environment, Buenos Aires, Argentina, CRC Press
Damek-Poprawa M, Sawicka-Kapusta K (2003) Damage to the liver, kidney, and testis with reference to burden of heavy metals in yellow-necked mice from areas around steelworks and zinc smelters in Poland. Toxicology 186:1–10
Mani U, Prasad AK, Kumar VS, Lal K, Kanojia RK, Chaudhari BP, Murthy RC (2007) Effect of fly ash inhalation on biochemical and histomorphological changes in rat liver. Ecotox Environ Safe 68:126–133
Liu JGRA, Goyer RA, Waalkes MP (2008) Chapter 23: toxic effects of metals in Casarett and Doull’s toxicology: the basic science of poisons. McGraw-Hill, New York
Wallenborn JG, Kovalcik KD, McGee JK, Landis MS, Kodavanti UP (2009) Systemic translocation of 70Zn: kinetics following intratracheal instillation in rats. Toxicol Appl Pharmacol 234:25–32
Sørensen M, Daneshvar B, Hansen M, Dragsted LO, Hertel O, Knudsen L, Loft S (2003) Personal PM2.5 exposure and markers of oxidative stress in blood. Environ Health Perspect 111:161
Papanikolaou G, Pantopoulos K (2005) Iron metabolism and toxicity. Toxicol Appl Pharmacol 202:199–211
Zhang W, Lei T, Lin ZQ, Zhang HS, Yang DF, Xi ZG et al (2011) Pulmonary toxicity study in rats with PM10 and PM2.5: differential responses related to scale and composition. Atmos Environ 45:1034–1041
Tan HH, Fiel MI, Sun Q, Guo J, Gordon RE, Chen LC et al (2009) Kupffer cell activation by ambient air particulate matter exposure may exacerbate non-alcoholic fatty liver disease. J Immunotoxicol 6:266–275
Xu J, Zhang W, Lu Z, Zhang F, Ding W (2017) Airborne PM2.5-induced hepatic insulin resistance by Nrf2/JNK-mediated signaling pathway. Int J Environ Res Public Health 14:787
Davidson RJ, Hamilton PJ (1978) High mean red cell volume: its incidence and significance in routine haematology. J Clin Pathol 31:493–498
Riediker M, Cascio WE, Griggs TR, Herbst MC, Bromberg PA, Neas L et al (2004) Particulate matter exposure in cars is associated with cardiovascular effects in healthy young men. Am J Respir Crit Care 169:934–940
Nalabotu SK, Kolli MB, Triest WE, Ma JY, Manne ND, Katta A et al (2011) Intratracheal instillation of cerium oxide nanoparticles induces hepatic toxicity in male Sprague-Dawley rats. Int J Nanomed 6:2327
Tomaru M, Takano H, Inoue KI, Yanagisawa R, Osakabe N, Yasuda A et al (2007) Pulmonary exposure to diesel exhaust particles enhances fatty change of the liver in obese diabetic mice. Int J Mol Med 19:17–22
Sharp P, Villano JS (2012) The laboratory rat. CRC Press, Boca Raton
Yeatts K, Svendsen E, Creason J, Alexis N, Herbst M, Scott J et al (2007) Coarse particulate matter (PM2.5–10) affects heart rate variability, blood lipids, and circulating eosinophils in adults with asthma. Environ Health Perspect 115:709
Chuang KJ, Yan YH, Cheng TJ (2010) Effect of air pollution on blood pressure, blood lipids, and blood sugar: a population-based approach. J Occup Environ Med 52:258–262
Conklin DJ (2013) From lung to liver: how does airborne particulate matter trigger NASH and systemic insulin resistance? J Hepatol 58:8–10
Vikram A, Jena G (2010) S961, an insulin receptor antagonist causes hyperinsulinemia, insulin-resistance and depletion of energy stores in rats. Biochem Biophys Res Commun 398:260–265
Sato S, Shirakawa H, Tomita S, Ohsaki Y, Haketa K, Tooi O et al (2008) Low-dose dioxins alter gene expression related to cholesterol biosynthesis, lipogenesis, and glucose metabolism through the aryl hydrocarbon receptor-mediated pathway in mouse liver. Toxicol Appl Pharmacol 229:10–19
Naem E, Alcalde R, Gladysz M, Mesliniene S, Jaimungal S, Sheikh-Ali M et al (2012) Inhibition of apolipoprotein AI gene by the aryl hydrocarbon receptor: a potential mechanism for smoking-associated hypoalphalipoproteinemia. Life Sci 91:64–69
Pei Y, Halbrook RS, Li H, You J (2017) Homing pigeons as a biomonitor for atmospheric PAHs and PCBs in Guangzhou, a megacity in South China. Mar Pollut Bull 124:1048–1054
Matsumoto ST, Mantovani MS, Malaguttii MIA, Dias AL, Fonseca IC, Marin-Morales MA (2006) Genotoxicity and mutagenicity of water contaminated with tannery effluents, as evaluated by the micronucleus test and comet assay using the fish Oreochromis niloticus and chromosome aberrations in onion root-tips. Genet Mol Biol 29:148–158
García-Lestón J, Méndez J, Pásaro E, Laffon B (2010) Genotoxic effects of lead: an updated review. Environ Int 36:623–636
Rodríguez-Mercado JJ, Altamirano-Lozano MA (2013) Genetic toxicology of thallium: a review. Drug Chem Toxicol 36:369–383
Anderson JO, Thundiyil JG, Stolbach A (2012) Clearing the air: a review of the effects of particulate matter air pollution on human health. J Med Toxicol 8:166–175
Calder PC, Dimitriadis G, Newsholme P (2007) Glucose metabolism in lymphoid and inflammatory cells and tissues. Curr Opin Clin Nutr 10:531–540
Moran LA, Horton HR, Scrimgeour KG, Perry MD, Rawn D (2013) Principles of biochemistry. Pearson New International Edition
Ramanathan G, Yin F, Speck M, Tseng CH, Brook JR, Silverman F et al (2015) Effects of urban fine particulate matter and ozone on HDL functionality. Part Fibre Toxicol 13:26
Mendez R, Zheng Z, Fan Z, Rajagopalan S, Sun Q, Zhang K (2013) Exposure to fine airborne particulate matter induces macrophage infiltration, unfolded protein response, and lipid deposition in white adipose tissue. Am J Trans Res 5:224
Jin Y, Miao W, Lin X, Wu T, Shen H, Chen S et al (2014) Sub-chronically exposing mice to a polycyclic aromatic hydrocarbon increases lipid accumulation in their livers. Environ Toxicol Pharmacol 38:353–363
Acknowledgements
We would like to thank Dr. Luis I. Juncos for his constructive comments and English language editing.
Funding
This work received partial support from Consejo Nacional de Investigaciones Científicas y Técnicas (Grant #11220090100999) and Secretaría de Ciencia y Técnica de la Universidad Nacional de Córdoba (Grant #30720110100529).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tavera Busso, I., Mateos, A.C., González Peroni, A. et al. Hepatic alterations associated with fine particulate matter exposure. Toxicol Res. 36, 139–148 (2020). https://doi.org/10.1007/s43188-019-00014-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43188-019-00014-4