Abstract
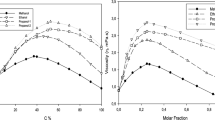
In this study, the hydrogen bonding term is used in combination with the Patel–Teja viscosity equation of state to model the viscosities of the mixtures of the alcohols. The pure-component parameters of the alcohols have been fitted according to the experimental data. Subsequently, an appropriate mixing rule has been suggested for the binary mixtures and new fitting parameters (BIPs) have been introduced. The cross association contributions have been used for the model, and it is extended to binary mixtures of alcohols. The accuracy of the present viscosity equation of state has been tested through the viscosities of 12 binary alcohol mixtures based on the deviations from the experimental data. Moreover, the model has been applied with and without binary interaction parameters. The results of this model show that the application of the application of binary interaction parameters has no significant effect on the accuracy of the model for primary linear alcohols while binary interaction parameters are required when isomers of the alcohols is present in the mixture. The overall average deviations of the viscosity are 15.49% and 3.87% without and with binary interaction parameters, respectively. Moreover, the calculations are done without hydrogen binding and it is found that the application of hydrogen bonding has no significant effect on the accuracy of the model.






Similar content being viewed by others
Data availability
There is no data obtained for this study.
Abbreviations
- a,b,c :
-
Parameters of PT equation of state
- e 0, e 1 :
-
Fitting parameters
- E :
-
Fitting parameter
- f :
-
Parameters of viscosity equation of state
- k ij :
-
Binary interaction parameter
- M :
-
Molecular mass, g/mol
- N :
-
Number of experimental points
- P :
-
Pressure, bar
- P c :
-
Critical pressure, bar
- P r :
-
Reduced pressure
- \(R^{\prime}\) :
-
Parameter of PT viscosity equation of state
- rc :
-
Parameter of PT viscosity equation of state
- T :
-
Temperature, K
- T c :
-
Critical temperature, K
- T d :
-
Specific temperature used to correct the computed viscosities, K
- \(T^{\prime}_{{}}\) :
-
Presumptive temperature, K
- \(T^{\prime}_{c}\) :
-
Presumptive critical temperature, K
- v :
-
Molar volume, cm3·mol−1
- \(v^{\prime}\) :
-
Fitting parameter
- x i :
-
Mole fraction of i in the liquid phase
- AAD%:
-
Average absolute deviation percent
- EOS:
-
Equation of State
- PT:
-
Patel–Teja
- α:
-
Fitting parameters
- β :
-
Parameter of viscosity equation of state
- η :
-
Adjusted critical compressibility factor
- \(\kappa\) :
-
Fitting parameters
- μ :
-
Viscosity, μPa·s
- μ c :
-
Critical viscosity, μPa·s
- Ωa,Ωb,Ωc,Ωac :
-
Parameters of PT equation of state
- ω :
-
Acentric factor
- c :
-
Critical
- calc :
-
Calculated
- exp :
-
Experimental
- r :
-
Reduced
References
Alam MDS, Baskar A, Siddiq AM (2018) The density, dynamic viscosity and kinematic viscosity of protic polar solvents (pure and mixed systems) studies: a theoretical insight of thermophysical properties. J Mol Liq 251:458–469. https://doi.org/10.1016/j.molliq.2017.12.089
Assael MJ, Polimatidou SK (1994) Measurements of the viscosity of alcohols in the temperature range 290–340 K at pressures up to 30 MPa. Int J Thermophys 15:95–107. https://doi.org/10.1007/BF01439248
Bravo-Sánchez MG, Iglesias-Silva GA, Estrada-Baltazar A, Hall KR (2010) Densities and viscosities of binary mixtures of n-butanol with 2-butanol, isobutanol, and tert-butanol from (303.15 to 343.15) K. J Chem Eng Data 55:2310–2315. https://doi.org/10.1021/je900722m
Cano-Gómez JJ, Iglesias-Silva GA, Ramos-Estrada M, Hall KR (2012) Densities and viscosities for binary liquid mixtures of ethanol + 1-propanol, 1-butanol, and 1-pentanol from (293.15 to 328.15) K at 0.1 MPa. J Chem Eng Data 57:2560–2567. https://doi.org/10.1021/je300632p
Cano-Gómez JJ, Iglesias-Silva GA, Rivas P, Díaz-Ovalle CO, Cerino-Córdova FDJ (2017) Densities and viscosities for binary liquid mixtures of biodiesel + 1-butanol, + isobutyl alcohol, or + 2-butanol from 293.15 to 333.15 K at 0.1 MPa. J Chem Eng Data 62:3391–3400. https://doi.org/10.1021/acs.jced.7b00440
Canosa J, Rodríguez A, Tojo J (1998) Dynamic viscosities of (methyl acetate or methanol) with (ethanol, 1-propanol, 2-propanol, 1-butanol, and 2-butanol) at 298.15 K. J Chem Eng Data 43:417–421. https://doi.org/10.1021/je9702302
Estrada-Baltazar A, Iglesias-Silva GA, Caballero-Cerón C (2013) Volumetric and transport properties of binary mixtures of n-octane + ethanol, + 1-propanol, + 1-butanol, and + 1-pentanol from (293.15 to 323.15) K at atmospheric pressure. J Chem Eng Data 58:3351–3363. https://doi.org/10.1021/je4004806
Fan T-B, Wang L-S (2006) A viscosity model based on Peng–Robinson equation of state for light hydrocarbon liquids and gases. Fluid Phase Equilib 247:59–69. https://doi.org/10.1016/j.fluid.2006.06.008
Feakins D, Bates FM, Waghorne WE, Lawrence KG (1993) Relative viscosities and quasi-thermodynamics of solutions of tert-butyl alcohol in the methanol–water system: a different view of the alkyl–water interaction. J Chem Soc Faraday Trans 89:3381–3388. https://doi.org/10.1039/FT9938903381
Giro F, Gonçalves M, Ferreira AG, Fonseca IM (2003) Viscosity and density data of the system water + n-pentyl acetate + methanol. Fluid Phase Equilib 204:217–232. https://doi.org/10.1016/S0378-3812(02)00262-5
Grunberg L, Nissan AH (1949) Mixture law for viscosity. Nature 164:799–800. https://doi.org/10.1038/164799b0
Guo X-Q, Wang L-S, Rong S-X, Guo T-M (1997) Viscosity model based on equations of state for hydrocarbon liquids and gases. Fluid Phase Equilib 139:405–421. https://doi.org/10.1016/S0378-3812(97)00156-8
Guo X-Q, Sun C-Y, Rong S-X, Chen G-J, Guo T-M (2000) Equation of state analog correlations for the viscosity and thermal conductivity of hydrocarbons and reservoir fluids. J Petrol Sci Eng 30:15–27. https://doi.org/10.1016/S0920-4105(01)00098-5
Habibi H, Hekmat-Nazemi A, Kamran-Pirzaman A, Mohammadi AH (2016) Modeling viscosity of alcohols based on the CPA-EoS + ƒ-theory. J Mol Liq 220:558–565. https://doi.org/10.1016/j.molliq.2016.04.052
Hironobu K, Sadahiro T, Masahiro M, Takeshi Y, Yoshiyuki T, Tadashi M (1980) Specific volume and viscosity of methanol-water mixtures under high pressure. Rev Phys Chem Jpn 49:59–69. http://hdl.handle.net/2433/47079.
Isdale JD, Easteal AJ, Woolf LA (1985) Shear viscosity of methanol and methanol + water mixtures under pressure. Int J Thermophys 6:439–450. https://doi.org/10.1007/BF00508889
Kadlec P, Henke S, Bubnik Z (2010) Properties of ethanol and ethanol-water solutions—tables and equations. Sugar Industry/Zuckerindustrie 135(2010):607–613. https://paperzz.com/doc/8999948.
Khosharay S (2014) Suggestion of mixing rule for parameters of PRμ model for light liquid hydrocarbon mixtures. Korean J Chem Eng 31:1246–1252. https://doi.org/10.1007/s11814-014-0043-1
Khosharay S, Karimi R, Khosharay K (2016) Modeling the viscosity for (nC5+nC8), (nC5+nC10), (nC8+nC10) and (nC5+nC8+nC10) systems with peng-robinson viscosity equation of state. Periodica Polytech, Chem Eng 60:259–265. https://doi.org/10.3311/PPch.8632
Khosharay S, Pierantozzi M, Di Nicola G (2018) Modeling investigation on the viscosity of pure refrigerants and their liquid mixtures by using the Patel–Teja viscosity equation of state. Int J Refrig 85:255–267. https://doi.org/10.1016/j.ijrefrig.2017.10.004
Kumagai A, Yokoyama C (1998) Liquid viscosity of binary mixtures of methanol with ethanol and 1-propanol from 273.15 to 333.15 K. Int J Thermophys 19:3–13. https://doi.org/10.1023/A:1021438800094
Macı́as-Salinas R, Garcı́a-Sánchez F, Eliosa-Jiménez G (2003) An equation-of-state-based viscosity model for non-ideal liquid mixtures. Fluid Phase Equilib 210:319–334https://doi.org/10.1016/S0378-3812(03)00169-9
Matsuo S, Makita T (1991) Viscosity of methanol and 2-methyl-2-propanol mixtures under high pressures. Int J Thermophys 12:459–468. https://doi.org/10.1007/BF00502362
Medeiros M, Téllez-Arredondo P (2008) Cubic two-state equation of state for associating fluids. Ind Eng Chem Res 47:5723–5733. https://doi.org/10.1021/ie071397j
Moha-Ouchane M, Boned C, Allal A, Benseddik M (1998) Viscosity and excess volume at high pressures in associative binaries. Int J Thermophys 19:161–189. https://doi.org/10.1023/A:1021455203728
Monsalvo MA, Baylaucq A, Quiñones-Cisneros SE, Boned C (2006) High-pressure viscosity behavior of x 1,1,1,2-tetrafluoroethane (HFC-134a)+(1–x) triethylene glycol dimethylether (TriEGDME) mixtures: measurements and modeling. Fluid Phase Equilib 247:70–79. https://doi.org/10.1016/j.fluid.2006.06.015
Nelder JA, Mead R (1965) A simplex method for function minimization. Compt J 7:308–313. https://doi.org/10.1093/comjnl/7.4.308
Niksirat M, Aeenjan F, Khosharay S (2021) Introducing hydrogen bonding term to the Patel-Teja viscosity equation of state for hydrochlorofluorocarbons, hydrofluorocarbons and hydrofluoroolefins. Fluid Phase Equilib 547:113178. https://doi.org/10.1016/j.fluid.2021.113178
Pang FM, Seng CE, Teng TT, Ibrahim MH (2007) Densities and viscosities of aqueous solutions of 1-propanol and 2-propanol at temperatures from 293.15 K to 333.15 K. J Mol Liq 136:71–78. https://doi.org/10.1016/j.molliq.2007.01.003
Patel NC, Teja AS (1982) A new cubic equation of state for fluids and fluid mixtures. Chem Eng Sci 37:463–473. https://doi.org/10.1016/0009-2509(82)80099-7
Quiñones-Cisneros SE, García J, Fernández J, Monsalvo MA (2005) Phase and viscosity behaviour of refrigerant–lubricant mixtures. Int J Refrig 28:714–724. https://doi.org/10.1016/j.ijrefrig.2004.12.004
Quiñones-Cisneros SE, Huber ML, Deiters UK (2012) Correlation for the viscosity of sulfur hexafluoride (SF6) from the triple point to 1000 K and pressures to 50 MPa. J Phys Chem Ref Data 41:23102–23111. https://doi.org/10.1063/1.3702441
Reid RC, Prausnitz JM, Poling BE (1897) The properties of gases and liquids
Tanaka Y, Matsuda Y, Fujiwara H, Kubota H, Makita T (1987) Viscosity of (water + alcohol) mixtures under high pressure. Int J Thermophys 8:147–163. https://doi.org/10.1007/BF00515199
Wang X, Wu J, Liu Z (2007) Viscosity modeling of several HFC refrigerants using the friction theory. Fluid Phase Equilib 262:251–263. https://doi.org/10.1016/j.fluid.2007.09.011
Xianga HW, Laesecke A, Huberb ML (2006) A new reference correlation for the viscosity of methanol. J Phys Chem Ref Data 35:1597. https://doi.org/10.1063/1.2360605
Yoshiyuki T, Takeshi Y, Yoshimasa S, Hironobu K, Tadashi M (1977) Specific volume and viscosity of ethanol-water mixtures under high pressure. Rev Phys Chem Jpn 47(1977):12–24. http://hdl.handle.net/2433/47042.
Author information
Authors and Affiliations
Contributions
MN: methodology, investigation, software, formal analysis, validation, writing original draft, writing—review and editing. FA: investigation, software, formal analysis, validation. SK: methodology, writing original draft, writing–review and editing, supervision.
Corresponding author
Ethics declarations
Conflict of interest
Author Mohammad niksirat declares that he/she has no conflict of interest. Author Fatemeh Aeenjan declares that he/she has no conflict of interest. Author Shahin Khosharay declares that he/she has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Niksirat, M., Aeenjan, F. & Khosharay, S. The application of the Patel–Teja viscosity equation of state for alcohols and their mixtures. Braz. J. Chem. Eng. 41, 585–595 (2024). https://doi.org/10.1007/s43153-023-00347-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43153-023-00347-6