Abstract
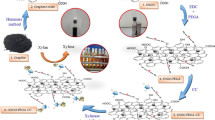
The present study demonstrates the coating of fullerenes with gluconic acid for the immobilization of Aspergillus oryzae β-galactosidase. The prepared nanomatrix provided 86% immobilization yield, and broadened the biocatalytic activity of immobilized enzyme at higher pH and temperature ranges. Immobilized β-galactosidase exhibited 60% activity even at 5.0% galactose concentration as compared to 23% enzyme activity obtained by soluble enzyme under similar experimental conditions. Reusability of the enzyme was improved considerably as a result of covalent immobilization. Immobilized β-galactosidase showed 89% activity even after sixth repeated use and could be recovered easily. The noticeable improvement in lactose hydrolysis was observed in batch reactors by immobilized enzyme in contrast to the soluble enzyme at high temperature ranges. At 50 °C, 89% lactose conversion was achieved by immobilized enzyme as compared to 77%, obtained by free β-galactosidase under similar incubation conditions. Hence, the developed immobilized enzyme preparation could be exploited for converting lactose into its monosaccharides in a convenient and cheaper way.





Similar content being viewed by others
Abbreviations
- GACF:
-
Gluconic acid coated fullerene
- IβG:
-
Immobilized β-galactosidase (β-galactosidase bound to GACF)
- NPs:
-
Nanoparticles
- SβG:
-
Soluble β-galactosidase
References
Alshanberi AM, Satar R, Ansari SA (2021) Stabilization of β-galactosidase on modified gold nanoparticles: a preliminary biochemical study to obtain lactose-free dairy products for lactose-intolerance individuals. Molecules 26(3):1226
Ansari SA, Husain Q (2010) Lactose hydrolysis by β galactosidase immobilized on concanavalin A-cellulose in batch and continuous mode. J Mol Catal B Enzym 63(1–2):68
Ansari SA, Husain Q (2012) Potential applications of enzymes immobilized on/in nanomaterials: a review. Biotechnol Adv 30(3):512
Ansari SA, Al-Shaeri M (2019) Biotechnological application of surface modified cerium oxide nanoparticles. Braz J Chem Eng 36(1):16
Ansari SA, Satar R, Zaidi SK, Naseer MI, Karim S, Alqahtani MH, Rasool M (2015a) Nanodiamonds as an effective and novel matrix for immobilizing β galactosidase. Food Bioprod Proc 95(4):298
Ansari SA, Satar R, Zaidi SK (2015b) Carboxylation of silver nanoparticles for the immobilization of β-galactosidase and its efficacy in galacto-oligosaccharides production. Quim Nova 38(3):58
Bohme DK (2016) Fullerene ion chemistry: a journey of discovery and achievement. Philos Trans Royal Soc A 374(2):321
Doane TL, Cheng Y, Babar A, Hill RJ, Burda C (2010) Electrophoretic mobilities of PEGylated gold NPs. J Am Chem Soc 132(44):15624
Guisan JM, Lopez-Gallego F, Bolivar JM, Rocha-Martin J, Fernandez-Lorente G (2020) The science of enzyme immobilization. Methods Mol Biol 2100(1):1
Guven RG, Kaplan A, Guven K, Matpan F, Dogru M (2011) Effects of various inhibitors on β-galactosidase purified from the thermoacidophilic Alicyclobacillus acidocaldarius subsp. Rittmannii isolated from Antarctica. Biotechnol Bioproc Eng 16(1):114
He H, Pham-Huy LA, Dramou P, Xiao D, Zuo P, Pham-Huy C (2013) Carbon nanotubes: applications in pharmacy and medicine. Biomed Res Internat 2013(5):578290
Kalska-Szostko B, Rogowska M (2012) Preparation of magnetite-fullerene nanocomposite with enzyme immobilization. J Nanosci Nanotechnol 12(9):6907
Khalid K, Tan X, Zaid HFM, Tao Y, Chew CL, Chu DT, Lam MK, Ho YC, Lim JW, Wei LC (2020) Advanced in developmental organic and inorganic nanomaterial: a review. Bioengineered 11(1):328
Moreno C, Divins NJ, Gazquez J, Varela M, Angurelle I, Llorca J (2012) Improved thermal stability of oxide-supported naked gold nanoparticles by ligand-assisted pinning. Nanoscale 4(7):2278
Panesar PS, Kumari S, Panesar R (2010) Potential applications of immobilized β-galactosidase in food processing industries. Enzyme Res 2(2):473
Pilehvar S, Wael KD (2015) Recent advances in electrochemical biosensors based on fullerene-C0 nano-structured platforms. Biosensors (basel) 5(4):712
Reis CLB, Sousa EYA, Serpa JF, Oliveira RC, Santos JCS (2019) Design of immobilized enzyme biocatalysts: drawbacks and opportunities. Quim Nova 42(7):13
Sami S, Etesami N (2017) Improving thermal characteristics and stability of phase change material containing TiO2 nanoparticles after thermal cycles for energy storage. Appl Therm Eng 124(3):346
Saqib S, Akram A, Halim SA, Tassaduq R (2017) Sources of β-galactosidase and its applications in food industry. 3 Biotech 7(1):79
Sastre DE, Reis EA, Netto CGCM (2020) Strategies to rationalize enzyme immobilization procedures. Methods Enzymol 630(2):81
Sui Y, Cui Y, Nie Y, Xia GM, Sun GX, Han JT (2012) Surface modification of magnetite nanoparticles using gluconic acid and their application in immobilized lipase. Colloid Surface B Biointerface 93(1):24
Ureta MM, Martins GN, Figueira O, Pires PF, Castilho PC, Gomez-Zavaglia A (2020) Recent advances in β-galactosidase and fructosyltransferase immobilization technology. Crit Rev Food Sci Nutr 32(4):1040
Wang EC, Wang AZ (2014) Nanoparticles and their applications in cell and molecular biology. Integrated Biology Cambridge 6(1):9
Wissing M, Niehues M, Ravoo BJ, Studer A (2020) Synthesis and immobilization of metal nanoparticles using photoactive polymer-decorated zeolite L crystals and their application in catalysis. Adv Synt Cataly 362(11):2245
Yang X, Ebrahimi A, Li J, Cui Q (2014) Fullerene-biomolecule conjugates and their biomedicinal applications. Int J Nanomed 9(1):77
Zhou QZK, Chen DC (2001) Effects of temperature and pH on the catalytic activity of the immobilized β-galactosidase from Kluyveromyces lactis. Biochem Eng J 9(1):33
Acknowledgements
Mr. Jitendra Kumar of Department of Biotechnology (Chaudhary Charan Singh University, India) is gratefully acknowledged for providing the fullerene used in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations
Rights and permissions
About this article
Cite this article
Ansari, S.A., Alshanberi, A.M. Stability studies of β-galactosidase immobilized on gluconic acid coated fullerenes. Braz. J. Chem. Eng. 39, 361–367 (2022). https://doi.org/10.1007/s43153-021-00146-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43153-021-00146-x