Abstract
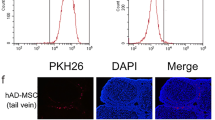
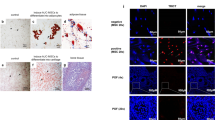
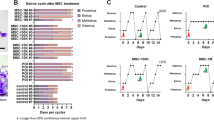
The efficacy of human amniotic mesenchymal stem cell (hAMSC) ovarian injection in improving ovarian function in primary ovarian insufficiency (POI) patients has been shown in some reports. However, the safety and efficacy of hAMSC vein injection remains unclear. In this study, we evaluated the safety and efficacy of hAMSC intravenous injection in cynomolgus macaques and SD rats and provided evidence for clinical trials. The hAMSCs were transplanted three times in SD rats at low, medium, and high doses. The animal behavior and biochemical and biophysical parameters were routinely monitored on a 2-month period posttransplantation, and histopathologic examinations were also performed. Experiments on the acute toxicity, allergy test, and hemolysis test showed that hAMSCs possess good biocompatibility. Our results showed that the maximum tolerated dose of hAMSCs in SD rats was 4.0 × 107 cells/kg. The maximum safe dose with three injections of hAMSCs in SD rats was 5.0 × 106 cells/kg. In addition, the results demonstrated that hAMSCs may restore POI rat ovarian function after two injections of 2.5 × 106 cells/kg or 5.0 × 106 cells/kg, which improved the disturbed estrous cycle, hormone levels, and ovarian lesions induced by pZP3. In conclusion, the preclinical results suggested that the transplantation of hAMSCs may be safe and efficacious for SD rats at doses of 5.0 × 106 cells/kg and lower.


Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Code Availability
Not applicable.
References
European Society for Human, R, et al. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31(5):926–37.
Mohamed SA, et al. Human mesenchymal stem cells partially reverse infertility in chemotherapy-induced ovarian failure. Reprod Sci. 2018;25(1):51–63.
Lai D, et al. Human amniotic fluid stem cells have a potential to recover ovarian function in mice with chemotherapy-induced sterility. BMC Dev Biol. 2013;13:34.
Kirshenbaum M, Orvieto R. Premature ovarian insufficiency (POI) and autoimmunity-an update appraisal. J Assist Reprod Genet. 2019;36(11):2207–15.
Ding L, et al. Transplantation of UC-MSCs on collagen scaffold activates follicles in dormant ovaries of POF patients with long history of infertility. Sci China Life Sci. 2018;61(12):1554–65.
Ling L, et al. Human amnion-derived mesenchymal stem cell (hAD-MSC) transplantation improves ovarian function in rats with premature ovarian insufficiency (POI) at least partly through a paracrine mechanism. Stem Cell Res Ther. 2019;10(1):46.
Ding C, et al. Different therapeutic effects of cells derived from human amniotic membrane on premature ovarian aging depend on distinct cellular biological characteristics. Stem Cell Res Ther. 2017;8(1):173.
Klein JD, Fauza DO. Amniotic and placental mesenchymal stem cell isolation and culture. Methods Mol Biol. 2011;698:75–88.
Hulla J E, Navarro L, Kruger C L, et al. Toxicity, subchronic and chronic. In: Wexler P (ed) Encyclopedia of toxicology, 3rd edn. Oxford: Academic Press; 2014. p. 626–633.
Kazezoglu C, Serin E. The effect of different blood drawing methods on hemolysis and test results from intravenous catheters used in emergency departments. Clin Lab. 2019;65(1). https://doi.org/10.7754/Clin.Lab.2018.180614.
Walls AF, Newman Taylor AJ, Longbottom JL. Allergy to guinea pigs: I. Allergenic activities of extracts derived from the pelt, saliva, urine and other sources. Clin Allergy. 1985;15(3):241–51.
Li J, et al. Treatment of autoimmune ovarian disease by co-administration with mouse zona pellucida protein 3 and DNA vaccine through induction of adaptive regulatory T cells. J Gene Med. 2008;10(7):810–20.
Mancuso L, Cao G. Acute toxicity test of CuO nanoparticles using human mesenchymal stem cells. Toxicol Mech Methods. 2014;24(7):449–54.
Liu A, et al. Therapeutic potential of mesenchymal stem/stromal cell-derived secretome and vesicles for lung injury and disease. Expert Opin Biol Ther. 2020;20(2):125–40.
Ding C, et al. Exosomal miRNA-320a is released from hAMSCs and regulates SIRT4 to prevent reactive oxygen species generation in POI. Mol Ther Nucleic Acids. 2020;21:37–50.
Pluchino N, Taylor HS. Endometriosis and stem cell trafficking. Reprod Sci. 2016;23(12):1616–9.
Salmeri N, Viganò P, Cavoretto P, Marci R, Candiani M. The kisspeptin system in and beyond reproduction: exploring intricate pathways and potential links between endometriosis and polycystic ovary syndrome. Rev Endocr Metab Disord. 2023. https://doi.org/10.1007/s11154-023-09826-0.
Yao X, et al. The paracrine effect of transplanted human amniotic epithelial cells on ovarian function improvement in a mouse model of chemotherapy-induced primary ovarian insufficiency. Stem Cells Int. 2016;2016:4148923.
Li Z, et al. Mesenchymal stem cells in premature ovarian insufficiency: mechanisms and prospects. Front Cell Dev Biol. 2021;9:718192.
Li X, et al. The effects of adenoviral transfection of the keratinocyte growth factor gene on epidermal stem cells: an in vitro study. Mol Cells. 2013;36(4):316–21.
Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: the role of anti-Mullerian hormone. Reproduction. 2002;124(5):601–9.
Wang J, Gao S, Zhao Y, Fan T, Zhang M, Chang D. Manufacture and quality control of human umbilical cord-derived mesenchymal stem cell sheets for clinical use. Cells. 2022;11(17):2732.
Acknowledgements
We thank the donors of stem cells, the Department of Obstetrics and Gynaecology in The Second Hospital of University of South China and Tianjin Tiancheng New Drug Evaluation.
Funding
This work was supported by Scientific and Technological Innovation Program of High-tech Industry of Hunan Province (NO: 2020GK4082), Changsha Key Research And Development Program (NO: kh2201247), Loudi Key Generic technologies Program (NO: Lou Caijiao No. 1 [2022]).
Author information
Authors and Affiliations
Contributions
Yuan Yang analyzed most of the data and wrote the initial draft of the paper; Li Li, Tenglong Yan, Jiangzhou Hua, Shiping Li, Yun Liu, and Sijie Yu analyzed the data and interpreted the results; Hongmei Zhang and Shihuan Tang acquired resources; Zhigang Xue, Xianping Zhang, and Chunbing Zheng conceived the idea of the study; all authors discussed the results and revised the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
The Animal Ethic Committee of Tianjin Tiancheng New Drug Evaluation approved the acute toxicity assay (NO: 2021120102), long toxicity and tissue distribution assay (NO: 2022022802), allergy test (NO: 2022051801), cynomolgus macaque assay (NO: 2021120102), POI rats assay (NO: 2022030703), and the Ethic Committee of the Second Hospital of University of South China approved the collection of amniotic membrane sample (NO: 202208–01).
Consent for Publication
Written informed consent for publication was obtained from all participants.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. hAMSC may restore POI rat ovarian function.
2. hAMSC did not cause haemolysis.
3. Multiple transplantation of hAMSC may be safe for SD rats at doses of 5.0 × 106 cells/kg and lower.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Y., Li, L., Yan, T. et al. Evaluation of Safety and Efficacy of Amniotic Mesenchymal Stem Cells for POI in Animals. Reprod. Sci. 31, 1159–1169 (2024). https://doi.org/10.1007/s43032-023-01417-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-023-01417-3