Abstract
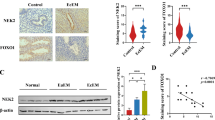
Endometriosis is a hormone-dependent disease associated with impaired immunoregulation. In our recent study, we have characterized the trascriptomic transformation of eutopic endometrium from patients with minimal/mild endometriosis and controls across the menstrual cycle. However, the regulatory mechanism of altered immune microenvironment in eutopic endometrial stromal cells (ESCs) remains unclear. Here, we want to explore the regulation of immune cell to progesterone resistance and endometrial receptivity in the eutopic ESCs by cytokine (TGF-β1), and to understand the effect of TGF-β1 on the decidualization of the eutopic ESCs. Primary culture of eutopic ESCs was performed to explore the effects of TGF-β1 on the expression of Smad and progesterone receptor (PR) and the in vitro decidualization. Additionally, co-immunoprecipitation (Co-IP) was used to explore the direct interaction between Smad and PR. We found an attenuate expression of PRB protein (p=0.026) after using TGF-β1 in eutopic ESCs, although the difference of PRA before and after treatment was not significant (p=0.678). Similarly, the results of qRT-PCR showed that the mRNA level of PR (p<0.001), PRB (p=0.003) and HOXA10 (p<0.001) decreased significantly after TGF-β1 treatment, but that increased (p<0.023, for all) after SB431542 treatment in the eutopic ESCs. Moreover, TGF-β1 has a negative effect on the in vitro decidualization of eutopic ESCs (p=0.003). And the group with treatment of both TGF-β1 and SB435142 in eutopic ESCs showed significant decidual-like changes with increased prolactin level (p=0.01). We did not observe any physical interaction between the PR and p-Smad3/Smad3 proteins by using Co-IP. By activating TGF-β/Smad signaling in eutopic ESCs, elevated TGF-β1 from CD45+ immune cells could attenuate expression of PR, and further decrease endometrial receptivity.






Similar content being viewed by others
Data Availability
The sing cell data of the current study are available in the NCBI’s Gene Expression Omnibus (accession code GSE214411).
References
Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. 2020;382(13):1244–56. https://doi.org/10.1056/NEJMra1810764.
The Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil Steril. 2012;98(3):591–8. https://doi.org/10.1016/j.fertnstert.2012.05.031.
Lessey BA, Kim JJ. Endometrial receptivity in the eutopic endometrium of women with endometriosis: it is affected, and let me show you why. Fertil Steril. 2017;108(1):19–27. https://doi.org/10.1016/j.fertnstert.2017.05.031.
Bafort C, Beebeejaun Y, Tomassetti C, Bosteels J, Duffy JM. Laparoscopic surgery for endometriosis. Cochrane Database Syst Rev. 2020;10(10):Cd011031. https://doi.org/10.1002/14651858.CD011031.pub3.
Marcoux S, Maheux R, Bérubé S. Laparoscopic surgery in infertile women with minimal or mild endometriosis. Canadian Collaborative Group on Endometriosis. N Engl J Med. 1997;337(4):217–22. https://doi.org/10.1056/nejm199707243370401.
Zhang X, Liu D, Huang W, Wang Q, Feng X, Tan J. Prediction of Endometriosis Fertility Index in patients with endometriosis-associated infertility after laparoscopic treatment. Reprod Biomed Online. 2018;37(1):53–9. https://doi.org/10.1016/j.rbmo.2018.03.012.
Kohl Schwartz AS, Wölfler MM, Mitter V, Rauchfuss M, Haeberlin F, Eberhard M, et al. Endometriosis, especially mild disease: a risk factor for miscarriages. Fertil Steril. 2017;108(5):806–14.e2. https://doi.org/10.1016/j.fertnstert.2017.08.025.
Valdes CT, Schutt A, Simon C. Implantation failure of endometrial origin: it is not pathology, but our failure to synchronize the developing embryo with a receptive endometrium. Fertil Steril. 2017;108(1):15–8. https://doi.org/10.1016/j.fertnstert.2017.05.033.
Lessey BA, Young SL. What exactly is endometrial receptivity? Fertil Steril. 2019;111(4):611–7. https://doi.org/10.1016/j.fertnstert.2019.02.009.
Brosens JJ, Hayashi N, White JO. Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinology. 1999;140(10):4809–20. https://doi.org/10.1210/endo.140.10.7070.
Kaya HS, Hantak AM, Stubbs LJ, Taylor RN, Bagchi IC, Bagchi MK. Roles of progesterone receptor A and B isoforms during human endometrial decidualization. Mol Endocrinol. 2015;29(6):882–95. https://doi.org/10.1210/me.2014-1363.
Patel B, Elguero S, Thakore S, Dahoud W, Bedaiwy M, Mesiano S. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum Reprod Update. 2015;21(2):155–73. https://doi.org/10.1093/humupd/dmu056.
Lu H, Yang X, Zhang Y, Lu R, Wang X. Epigenetic disorder may cause downregulation of HOXA10 in the eutopic endometrium of fertile women with endometriosis. Reprod Sci. 2013;20(1):78–84. https://doi.org/10.1177/1933719112451146.
Schmitz CR, Oehninger S, Genro VK, Chandra N, Lattanzio F, Yu L, et al. Alterations in expression of endometrial milk fat globule-EGF factor 8 (MFG-E8) and leukemia inhibitory factor (LIF) in patients with infertility and endometriosis. JBRA Assist Reprod. 2017;21(4):313–20. https://doi.org/10.5935/1518-0557.20170056.
Patel BG, Rudnicki M, Yu J, Shu Y, Taylor RN. Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstet Gynecol Scand. 2017;96(6):623–32. https://doi.org/10.1111/aogs.13156.
Marquardt RM, Kim TH, Shin JH, Jeong JW. Progesterone and estrogen signaling in the endometrium: what goes wrong in endometriosis? Int J Mol Sci. 2019;20(15). https://doi.org/10.3390/ijms20153822.
Wu Y, Strawn E, Basir Z, Halverson G, Guo SW. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in endometriosis. Epigenetics. 2006;1(2):106–11. https://doi.org/10.4161/epi.1.2.2766.
Zelenko Z, Aghajanova L, Irwin JC, Giudice LC. Nuclear receptor, coregulator signaling, and chromatin remodeling pathways suggest involvement of the epigenome in the steroid hormone response of endometrium and abnormalities in endometriosis. Reprod Sci. 2012;19(2):152–62. https://doi.org/10.1177/1933719111415546.
Zhou M, Fu J, Xiao L, Yang S, Song Y, Zhang X, et al. miR-196a overexpression activates the MEK/ERK signal and represses the progesterone receptor and decidualization in eutopic endometrium from women with endometriosis. Hum Reprod. 2016;31(11):2598–608. https://doi.org/10.1093/humrep/dew223.
Pei T, Liu C, Liu T, Xiao L, Luo B, Tan J, et al. miR-194-3p Represses the progesterone receptor and decidualization in eutopic endometrium from women with endometriosis. Endocrinology. 2018;159(7):2554–62. https://doi.org/10.1210/en.2018-00374.
Lessey BA, Lebovic DI, Taylor RN. Eutopic endometrium in women with endometriosis: ground zero for the study of implantation defects. Semin Reprod Med. 2013;31(2):109–24. https://doi.org/10.1055/s-0032-1333476.
Vannuccini S, Clifton VL, Fraser IS, Taylor HS, Critchley H, Giudice LC, et al. Infertility and reproductive disorders: impact of hormonal and inflammatory mechanisms on pregnancy outcome. Hum Reprod Update. 2016;22(1):104–15. https://doi.org/10.1093/humupd/dmv044.
Casslén B, Sandberg T, Gustavsson B, Willén R, Nilbert M. Transforming growth factor beta1 in the human endometrium. Cyclic variation, increased expression by estradiol and progesterone, and regulation of plasminogen activators and plasminogen activator inhibitor-1. Biol Reprod. 1998;58(6):1343–50. https://doi.org/10.1095/biolreprod58.6.1343.
Kim MR, Park DW, Lee JH, Choi DS, Hwang KJ, Ryu HS, et al. Progesterone-dependent release of transforming growth factor-beta1 from epithelial cells enhances the endometrial decidualization by turning on the Smad signalling in stromal cells. Mol Hum Reprod. 2005;11(11):801–8. https://doi.org/10.1093/molehr/gah240.
Oosterlynck DJ, Meuleman C, Waer M, Koninckx PR. Transforming growth factor-beta activity is increased in peritoneal fluid from women with endometriosis. Obstet Gynecol. 1994;83(2):287–92.
Chegini N, Gold LI, Williams RS. Localization of transforming growth factor beta isoforms TGF-beta 1, TGF-beta 2, and TGF-beta 3 in surgically induced endometriosis in the rat. Obstet Gynecol. 1994;83(3):455–61.
Ma L, Andrieu T, McKinnon B, Duempelmann L, Peng RW, Wotzkow C, et al. Epithelial-to-mesenchymal transition contributes to the downregulation of progesterone receptor expression in endometriosis lesions. J Steroid Biochem Mol Biol. 2021;212:105943. https://doi.org/10.1016/j.jsbmb.2021.105943.
Vallve-Juanico J, Houshdaran S, Giudice LC. The endometrial immune environment of women with endometriosis. Hum Reprod Update. 2019;25(5):564–91. https://doi.org/10.1093/humupd/dmz018.
Canis M, Donnez JG, Guzick DS, Halme JK, Rock JA, Schenken RS, Vernon MW. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67(5):817–21. https://doi.org/10.1016/s0015-0282(97)81391-x.
Huang X, Wu L, Pei T, Liu D, Liu C, Luo B, et al. Single-cell transcriptome analysis reveals endometrial immune microenvironment in minimal/mild endometriosis. Clin Exp Immunol. 2023;212(3):285–95. https://doi.org/10.1093/cei/uxad029.
Lachapelle MH, Hemmings R, Roy DC, Falcone T, Miron P. Flow cytometric evaluation of leukocyte subpopulations in the follicular fluids of infertile patients. Fertil Steril. 1996;65(6):1135–40. https://doi.org/10.1016/s0015-0282(16)58327-7.
Bunis DG, Wang W, Vallvé-Juanico J, Houshdaran S, Sen S, Ben Soltane I, et al. Whole-tissue deconvolution and scRNAseq analysis identify altered endometrial cellular compositions and functionality associated with endometriosis. Front Immunol. 2021;12:788315. https://doi.org/10.3389/fimmu.2021.788315.
Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci. 2011;1221(1):80–7. https://doi.org/10.1111/j.1749-6632.2010.05938.x.
Saito S, Tsukaguchi N, Hasegawa T, Michimata T, Tsuda H, Narita N. Distribution of Th1, Th2, and Th0 and the Th1/Th2 cell ratios in human peripheral and endometrial T cells. Am J Reprod Immunol. 1999;42(4):240–5. https://doi.org/10.1111/j.1600-0897.1999.tb00097.x.
Chen S, Zhang J, Huang C, Lu W, Liang Y, Wan X. Expression of the T regulatory cell transcription factor FoxP3 in peri-implantation phase endometrium in infertile women with endometriosis. Reprod Biol Endocrinol. 2012;10:34. https://doi.org/10.1186/1477-7827-10-34.
Ingman WV, Robertson SA. Defining the actions of transforming growth factor beta in reproduction. Bioessays. 2002;24(10):904–14. https://doi.org/10.1002/bies.10155.
Itoh H, Kishore AH, Lindqvist A, Rogers DE, Word RA. Transforming growth factor β1 (TGFβ1) and progesterone regulate matrix metalloproteinases (MMP) in human endometrial stromal cells. J Clin Endocrinol Metab. 2012;97(6):E888–97. https://doi.org/10.1210/jc.2011-3073.
Kane N, Jones M, Brosens JJ, Saunders PT, Kelly RW, Critchley HO. Transforming growth factor-beta1 attenuates expression of both the progesterone receptor and Dickkopf in differentiated human endometrial stromal cells. Mol Endocrinol. 2008;22(3):716–28. https://doi.org/10.1210/me.2007-0316.
Kim JJ, Taylor HS, Lu Z, Ladhani O, Hastings JM, Jackson KS, et al. Altered expression of HOXA10 in endometriosis: potential role in decidualization. Mol Hum Reprod. 2007;13(5):323–32. https://doi.org/10.1093/molehr/gam005.
Fambrini M, Sorbi F, Bussani C, Cioni R, Sisti G, Andersson KL. Hypermethylation of HOXA10 gene in mid-luteal endometrium from women with ovarian endometriomas. Acta Obstet Gynecol Scand. 2013;92(11):1331–4. https://doi.org/10.1111/aogs.12236.
Doherty LF, Taylor HS. Leiomyoma-derived transforming growth factor-β impairs bone morphogenetic protein-2-mediated endometrial receptivity. Fertil Steril. 2015;103(3):845–52. https://doi.org/10.1016/j.fertnstert.2014.12.099.
Vićovac LM, Starkey PM, Aplin JD. Comment: effect of cytokines on prolactin production by human decidual stromal cells in culture: studies using cells freed of bone marrow-derived contaminants. J Clin Endocrinol Metab. 1994;79(6):1877–82. https://doi.org/10.1210/jcem.79.6.7989496.
Mazella J, Tang M, Tseng L. Disparate effects of relaxin and TGFbeta1: relaxin increases, but TGFbeta1 inhibits, the relaxin receptor and the production of IGFBP-1 in human endometrial stromal/decidual cells. Hum Reprod. 2004;19(7):1513–8. https://doi.org/10.1093/humrep/deh274.
Coya R, Alvarez CV, Perez F, Gianzo C, Diéguez C. Effects of TGF-beta1 on prolactin synthesis and secretion: an in-vitro study. J Neuroendocrinol. 1999;11(5):351–60. https://doi.org/10.1046/j.1365-2826.1999.00336.x.
Stoikos CJ, Harrison CA, Salamonsen LA, Dimitriadis E. A distinct cohort of the TGFbeta superfamily members expressed in human endometrium regulate decidualization. Hum Reprod. 2008;23(6):1447–56. https://doi.org/10.1093/humrep/den110.
Thackray VG, Mellon PL. Synergistic induction of follicle-stimulating hormone beta-subunit gene expression by gonadal steroid hormone receptors and Smad proteins. Endocrinology. 2008;149(3):1091–102. https://doi.org/10.1210/en.2007-1498.
Acknowledgements
We are grateful to all study participants, doctors, and operating room nurses at West China Second University Hospital of Sichuan University.
Funding
This study was supported by the National Natural Science Foundation of China (Grant number: 82071625).
Author information
Authors and Affiliations
Contributions
LW designed and performed the experiments and analyzed data, drafted the manuscript. XH designed and performed the experiments and analyzed data, and revised the manuscript. RW and JL assisted with experiments and analyzed data. HZ and YO contributed to sample collection and supervised the research. WH designed and supervised the research, revised the manuscript, and approved the final vision.
Corresponding author
Ethics declarations
Ethics Approval
The study was approved by the Ethics Committee of West China second University Hospital of Sichuan University (Grant number:2021028).
Consent to Participate
Written informed consents were obtained from all participants.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, L., Huang, X., Wang, R. et al. Increased Expression of TGF-β1 Contributes to the Downregulation of Progesterone Receptor Expression in the Eutopic Endometrium of Infertile Women with Minimal/Mild Endometriosis. Reprod. Sci. 30, 3578–3589 (2023). https://doi.org/10.1007/s43032-023-01315-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-023-01315-8