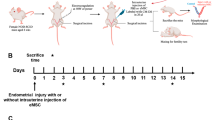
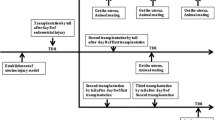
Abstract
Endometrial injury is one of the leading causes of female infertility and is caused by intrauterine surgery, endometrial infection, repeated abortion, or genital tuberculosis. Currently, there is little effective treatment to restore the fertility of patients with severe intrauterine adhesions and thin endometrium. Recent studies have confirmed the promising therapeutic effects of mesenchymal stem cell transplantation on various diseases with definite tissue injury. The aim of this study is to investigate the improvements of menstrual blood-derived endometrial stem cells (MenSCs) transplantation on functional restoration in the endometrium of mouse model. Therefore, ethanol-induced endometrial injury mouse models were randomly divided into two groups: the PBS-treated group, and the MenSCs-treated group. As expected, the endometrial thickness and gland number in the endometrium of MenSCs-treated mice were significantly improved compared to those of PBS-treated mice (P < 0.05), and fibrosis levels were significantly reduced (P < 0.05). Subsequent results revealed that MenSCs treatment significantly promoted angiogenesis in the injured endometrium. Simultaneously, MenSCs enhance the proliferation and antiapoptotic capacity of endometrial cells, which is likely contributed by activating the PI3K/Akt signaling pathway. Further tests also confirmed the chemotaxis of GFP-labeled MenSCs towards the injured uterus. Consequently, MenSCs treatment significantly improved the pregnant mice and the number of embryos in pregnant mice. This study confirmed the superior improvements of MenSCs transplantation on the injured endometrium and uncovered the potential therapeutic mechanism, which provides a promising alternative for patients with serious endometrial injury.







Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Code Availability
Not applicable.
References
Azizi R, Aghebati-Maleki L, Nouri M, Marofi F, Negargar S, Yousefi M. Stem cell therapy in Asherman syndrome and thin endometrium: Stem cell- based therapy. Biomed Pharmacother. 2018;102:333–43.
Miwa I, Tamura H, Takasaki A, Yamagata Y, Shimamura K, Sugino N. Pathophysiologic features of “thin” endometrium. Fertil Steril. 2009;91(4):998–1004.
Kou L, Jiang X, Xiao S, Zhao YZ, Yao Q, Chen R. Therapeutic options and drug delivery strategies for the prevention of intrauterine adhesions. J Control Release. 2020;318:25–37.
Yokomizo R, Fujiki Y, Kishigami H, Kishi H, Kiyono T, Nakayama S, et al. Endometrial regeneration with endometrial epithelium: homologous orchestration with endometrial stroma as a feeder. Stem Cell Res Ther. 2021;12(1):130.
Hooker AB, Lemmers M, Thurkow AL, Heymans MW, Opmeer BC, Brolmann HA, et al. Systematic review and meta-analysis of intrauterine adhesions after miscarriage: prevalence, risk factors and long-term reproductive outcome. Hum Reprod Update. 2014;20(2):262–78.
Hooker A, Fraenk D, Brolmann H, Huirne J. Prevalence of intrauterine adhesions after termination of pregnancy: a systematic review. Eur J Contracept Reprod Health Care. 2016;21(4):329–35.
Mentula M, Mannisto J, Gissler M, Heikinheimo O, Niinimaki M. Intrauterine adhesions following an induced termination of pregnancy: a nationwide cohort study. BJOG. 2018;125(11):1424–31.
Mahajan N, Sharma S. The endometrium in assisted reproductive technology: How thin is thin? J Hum Reprod Sci. 2016;9(1):3–8.
Lebovitz O, Orvieto R. Treating patients with “thin” endometrium - an ongoing challenge. Gynecol Endocrinol. 2014;30(6):409–14.
Bosteels J, van Wessel S, Weyers S, Broekmans FJ, D’Hooghe TM, Bongers MY, et al. Hysteroscopy for treating subfertility associated with suspected major uterine cavity abnormalities. Cochrane Database Syst Rev. 2018;12(12):CD009461.
Healy MW, Schexnayder B, Connell MT, Terry N, DeCherney AH, Csokmay JM, et al. Intrauterine adhesion prevention after hysteroscopy: a systematic review and meta-analysis. Am J Obstet Gynecol. 2016;215(3):267-75 e7.
Johary J, Xue M, Zhu X, Xu D, Velu PP. Efficacy of estrogen therapy in patients with intrauterine adhesions: systematic review. J Minim Invasive Gynecol. 2014;21(1):44–54.
Dreisler E, Kjer JJ. Asherman’s syndrome: current perspectives on diagnosis and management. Int J Womens Health. 2019;11:191–8.
Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726–36.
Chapel A, Bertho JM, Bensidhoum M, Fouillard L, Young RG, Frick J, et al. Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J Gene Med. 2003;5(12):1028–38.
Yu J, Jiang L, Gao Y, Sun Q, Liu B, Hu Y, et al. Interaction between BMSCs and EPCs promotes IUA angiogenesis via modulating PI3K/Akt/Cox2 axis. Am J Transl Res. 2018;10(12):4280–9.
Cao Y, Sun H, Zhu H, Zhu X, Tang X, Yan G, et al. Allogeneic cell therapy using umbilical cord MSCs on collagen scaffolds for patients with recurrent uterine adhesion: a phase I clinical trial. Stem Cell Res Ther. 2018;9(1):192.
Shao X, Ai G, Wang L, Qin J, Li Y, Jiang H, et al. Adipose-derived stem cells transplantation improves endometrial injury repair. Zygote. 2019;27(6):367–74.
Li B, Zhang Q, Sun J, Lai D. Human amniotic epithelial cells improve fertility in an intrauterine adhesion mouse model. Stem Cell Res Ther. 2019;10(1):257.
Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. 2016;7(1):125.
Hennes A, Devroe J, De Clercq K, Ciprietti M, Held K, Luyten K, et al. Protease secretions by the invading blastocyst induce calcium oscillations in endometrial epithelial cells via the protease-activated receptor 2. Reprod Biol Endocrinol. 2023;21(1):37.
Singh P, Bhartiya D. Mouse uterine stem cells are affected by endocrine disruption and initiate uteropathies. Reproduction. 2023;165(3):249–68.
Calle A, Lopez-Martin S, Monguio-Tortajada M, Borras FE, Yanez-Mo M, Ramirez MA. Bovine endometrial MSC: mesenchymal to epithelial transition during luteolysis and tropism to implantation niche for immunomodulation. Stem Cell Res Ther. 2019;10(1):23.
Navarrete F, Saravia F, Cisterna G, Rojas F, Silva PP, Rodriguez-Alvarez L, et al. Assessment of the anti-inflammatory and engraftment potential of horse endometrial and adipose mesenchymal stem cells in an in vivo model of post breeding induced endometritis. Theriogenology. 2020;155:33–42.
Liu Y, Niu R, Yang F, Yan Y, Liang S, Sun Y, et al. Biological characteristics of human menstrual blood-derived endometrial stem cells. J Cell Mol Med. 2018;22(3):1627–39.
Zhang S, Sun Y, Jiang D, Chen T, Liu R, Li X, et al. Construction and Optimization of an Endometrial Injury Model in Mice by Transcervical Ethanol Perfusion. Reprod Sci. 2021;28(3):693–702.
Xiao S, Wan Y, Xue M, Zeng X, Xiao F, Xu D, et al. Etiology, treatment, and reproductive prognosis of women with moderate-to-severe intrauterine adhesions. Int J Gynaecol Obstet. 2014;125(2):121–4.
Santamaria X, Cabanillas S, Cervello I, Arbona C, Raga F, Ferro J, et al. Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman’s syndrome and endometrial atrophy: a pilot cohort study. Hum Reprod. 2016;31(5):1087–96.
Zheng SX, Wang J, Wang XL, Ali A, Wu LM, Liu YS. Feasibility analysis of treating severe intrauterine adhesions by transplanting menstrual blood-derived stem cells. Int J Mol Med. 2018;41(4):2201–12.
Zhu H, Jiang Y, Pan Y, Shi L, Zhang S. Human menstrual blood-derived stem cells promote the repair of impaired endometrial stromal cells by activating the p38 MAPK and AKT signaling pathways. Reprod Biol. 2018;18(3):274–81.
Zhang Y, Lin X, Dai Y, Hu X, Zhu H, Jiang Y, et al. Endometrial stem cells repair injured endometrium and induce angiogenesis via AKT and ERK pathways. Reproduction. 2016;152(5):389–402.
Hu J, Song K, Zhang J, Zhang Y, Tan BZ. Effects of menstrual blood-derived stem cells on endometrial injury repair. Mol Med Rep. 2019;19(2):813–20.
Chen X, Wu Y, Wang Y, Chen L, Zheng W, Zhou S, et al. Human menstrual blood-derived stem cells mitigate bleomycin-induced pulmonary fibrosis through anti-apoptosis and anti-inflammatory effects. Stem Cell Res Ther. 2020;11(1):477.
Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene. 2003;22(56):8983–98.
Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21(1):92–101.
Zhang S, Li P, Yuan Z, Tan J. Platelet-rich plasma improves therapeutic effects of menstrual blood-derived stromal cells in rat model of intrauterine adhesion. Stem Cell Res Ther. 2019;10(1):61.
Ma H, Liu M, Li Y, Wang W, Yang K, Lu L, et al. Intrauterine transplantation of autologous menstrual blood stem cells increases endometrial thickness and pregnancy potential in patients with refractory intrauterine adhesion. J Obstet Gynaecol Res. 2020;46(11):2347–55.
Tan J, Li P, Wang Q, Li Y, Li X, Zhao D, et al. Autologous menstrual blood-derived stromal cells transplantation for severe Asherman’s syndrome. Hum Reprod. 2016;31(12):2723–9.
Zafardoust S, Kazemnejad S, Darzi M, Fathi-Kazerooni M, Rastegari H, Mohammadzadeh A. Improvement of Pregnancy rate and live birth rate in poor ovarian responders by intraovarian administration of autologous menstrual blood derived-mesenchymal stromal cells: phase I/II clinical trial. Stem Cell Rev Rep. 2020;16(4):755–63.
Weidt C, Niggemann B, Kasenda B, Drell TL, Zanker KS, Dittmar T. Stem cell migration: a quintessential stepping stone to successful therapy. Curr Stem Cell Res Ther. 2007;2(1):89–103.
Wang S, Wang Y, Xu B, Qin T, Lv Y, Yan H, et al. Biodistribution of 89Zr-oxine-labeled human bone marrow-derived mesenchymal stem cells by micro-PET/computed tomography imaging in Sprague-Dawley rats. Nucl Med Commun. 2022;43(7):834–46.
Togel F, Yang Y, Zhang P, Hu Z, Westenfelder C. Bioluminescence imaging to monitor the in vivo distribution of administered mesenchymal stem cells in acute kidney injury. Am J Physiol Renal Physiol. 2008;295(1):F315–21.
Francois M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20(1):187–95.
Alcayaga-Miranda F, Cuenca J, Luz-Crawford P, Aguila-Diaz C, Fernandez A, Figueroa FE, et al. Characterization of menstrual stem cells: angiogenic effect, migration and hematopoietic stem cell support in comparison with bone marrow mesenchymal stem cells. Stem Cell Res Ther. 2015;6(1):32.
Acknowledgements
We acknowledge all the authors for their contribution to the study.
Funding
We would like to acknowledge the Henan Province Foundation of China (212102310611 and 22A320043) and Xinxiang Foundation of China (GG2021029) for the financial support.
Author information
Authors and Affiliations
Contributions
J. L. and Y. L. conceived and designed the experiments. S. Z. and Y. P. analyzed the data and prepared the manuscript. R. Z. and Y. L. performed the experiments. H. C. provided assistance with cell culture and data analysis, and discussed the results and commented on the manuscript. S. Z. wrote the manuscript and prepared the figures; Y. L. revised the manuscript, and gave approval to the final version as the corresponding author. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
The present study was approved by the ethical committee of Xinxiang Medical University, Henan, China.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, S., Zhang, R., Yin, X. et al. MenSCs Transplantation Improve the Viability of Injured Endometrial Cells Through Activating PI3K/Akt Pathway. Reprod. Sci. 30, 3325–3338 (2023). https://doi.org/10.1007/s43032-023-01282-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-023-01282-0