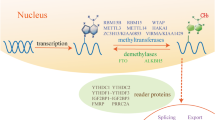
Abstract
Recently, epitranscriptional modification of N6-methyladenosine (m6A) has received growing attention in the research on the pathogenesis of preeclampsia. Advances in m6A sequencing have revealed the molecular mechanism and importance of m6A modification. In addition, epitranscriptional modification of m6A is closely related to the metabolic processes of placental tissues and cells in preeclampsia. This article reviews the composition, mode of action, and bioinformatics analysis of m6A modification-related proteins, and their biological function in the progression of preeclampsia. The relationship between m6A modification and preeclampsia risk factors, such as diabetes, cardiovascular disease, obesity, and psychological stress, is summarized to provide new ideas for studying PE-targeting molecules.


Similar content being viewed by others
References
Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2013;170:1–7.
Opichka MA, Rappelt MW, Gutterman DD, Grobe JL, McIntosh JJ. Vascular dysfunction in preeclampsia. Cells. 2021;10(11):3055.
Poon LC, Shennan A, Hyett JA, Kapur A, Hadar E, Divakar H, McAuliffe F, Da SCF, von Dadelszen P, McIntyre HD, Kihara AB, Di Renzo GC, Romero R, D’Alton M, Berghella V, Nicolaides KH, Hod M. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int J Gynaecol Obstet. 2019;145(Suppl 1):1–33.
Phipps EA, Thadhani R, Benzing T, Karumanchi SA. Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nat Rev Nephrol. 2019;15:275–89.
Boccaletto P, Machnicka MA, Purta E, Piatkowski P, Baginski B, Wirecki TK, de Crecy-Lagard V, Ross R, Limbach PA, Kotter A, Helm M, Bujnicki JM. MODOMICS: a database of RNA modification pathways. 2017 update. Nucleic Acids Res. 2018;46:D303–7.
Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, Yi C, Lindahl T, Pan T, Yang YG, He C. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7:885–7.
Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M, Sorek R, Rechavi G. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–6.
Han J, Wang JZ, Yang X, Yu H, Zhou R, Lu HC, Yuan WB, Lu JC, Zhou ZJ, Lu Q, Wei JF, Yang H. METTL3 promote tumor proliferation of bladder cancer by accelerating pri-miR221/222 maturation in m6A-dependent manner. Mol Cancer. 2019;18:110.
Li Z, Peng Y, Li J, Chen Z, Chen F, Tu J, Lin S, Wang H. N (6)-methyladenosine regulates glycolysis of cancer cells through PDK4. Nat Commun. 2020;11:2578.
Li Q, Ni Y, Zhang L, Jiang R, Xu J, Yang H, Hu Y, Qiu J, Pu L, Tang J, Wang X. HIF-1alpha-induced expression of m6A reader YTHDF1 drives hypoxia-induced autophagy and malignancy of hepatocellular carcinoma by promoting ATG2A and ATG14 translation. Signal Transduct Target Ther. 2021;6:76.
Zhao F, Xu Y, Gao S, Qin L, Austria Q, Siedlak SL, Pajdzik K, Dai Q, He C, Wang W, O’Donnell JM, Tang B, Zhu X. METTL3-dependent RNA m (6)A dysregulation contributes to neurodegeneration in Alzheimer’s disease through aberrant cell cycle events. Mol Neurodegener. 2021;16:70.
Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, Fu Y, Parisien M, Dai Q, Jia G, Ren B, Pan T, He C. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505:117–20.
Liu N, Dai Q, Zheng G, He C, Parisien M, Pan T. N (6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature. 2015;518:560–4.
Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, Weng X, Chen K, Shi H, He C. N(6)-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell. 2015;161:1388–99.
Zhang Y, Kang M, Zhang B, Meng F, Song J, Kaneko H, Shimamoto F, Tang B. m(6)A modification-mediated CBX8 induction regulates stemness and chemosensitivity of colon cancer via upregulation of LGR5. Mol Cancer. 2019;18:185.
Liu Z, Wang T, She Y, Wu K, Gu S, Li L, Dong C, Chen C, Zhou Y. N (6)-methyladenosine-modified circIGF2BP3 inhibits CD8(+) T-cell responses to facilitate tumor immune evasion by promoting the deubiquitination of PD-L1 in non-small cell lung cancer. Mol Cancer. 2021;20:105.
Kumari N, Karmakar A, Ahamad KM, Ganesan SK. The potential role of m6A RNA methylation in diabetic retinopathy. Exp Eye Res. 2021;208:108616.
Li X, Xiong W, Long X, Dai X, Peng Y, Xu Y, Zhang Z, Zhang L, Liu Y. Inhibition of METTL3/m6A/miR126 promotes the migration and invasion of endometrial stromal cells in endometriosisdagger. Biol Reprod. 2021;105:1221–33.
Bokar JA, Shambaugh ME, Polayes D, Matera AG, Rottman FM. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA. 1997;3:1233–47.
Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X, Dai Q, Chen W, He C. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10:93–5.
Agarwala SD, Blitzblau HG, Hochwagen A, Fink GR. RNA methylation by the MIS complex regulates a cell fate decision in yeast. PLoS Genet. 2012;8:e1002732.
Pendleton KE, Chen B, Liu K, Hunter OV, Xie Y, Tu BP, Conrad NK. The U6 snRNA m(6)A Methyltransferase METTL16 Regulates SAM Synthetase Intron Retention. Cell. 2017;169:824–35.
Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ, Vagbo CB, Shi Y, Wang WL, Song SH, Lu Z, Bosmans RP, Dai Q, Hao YJ, Yang X, Zhao WM, Tong WM, Wang XJ, Bogdan F, Furu K, Fu Y, Jia G, Zhao X, Liu J, Krokan HE, Klungland A, Yang YG, He C. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49:18–29.
Zhao X, Yang Y, Sun BF, Shi Y, Yang X, Xiao W, Hao YJ, Ping XL, Chen YS, Wang WJ, Jin KX, Wang X, Huang CM, Fu Y, Ge XM, Song SH, Jeong HS, Yanagisawa H, Niu Y, Jia GF, Wu W, Tong WM, Okamoto A, He C, Rendtlew DJ, Wang XJ, Yang YG. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res. 2014;24:1403–19.
Liu H, Wang H, Wei Z, Zhang S, Hua G, Zhang SW, Zhang L, Gao SJ, Meng J, Chen X, Huang Y. MeT-DB V2.0: elucidating context-specific functions of N6-methyl-adenosine methyltranscriptome. Nucleic Acids Res. 2018;46:D281–7.
Liu Q, Gregory RI. RNAmod: an integrated system for the annotation of mRNA modifications. Nucleic Acids Res. 2019;47:W548–55.
Xuan JJ, Sun WJ, Lin PH, Zhou KR, Liu S, Zheng LL, Qu LH, Yang JH. RMBase v2.0: deciphering the map of RNA modifications from epitranscriptome sequencing data. Nucleic Acids Res. 2018;46:D327–34.
Zheng Y, Nie P, Peng D, He Z, Liu M, Xie Y, Miao Y, Zuo Z, Ren J. m6AVar: a database of functional variants involved in m6A modification. Nucleic Acids Res. 2018;46:D139–45.
Tang Y, Chen K, Song B, Ma J, Wu X, Xu Q, Wei Z, Su J, Liu G, Rong R, Lu Z, de Magalhaes JP, Rigden DJ, Meng J. m6A-Atlas: a comprehensive knowledgebase for unraveling the N6-methyladenosine (m6A) epitranscriptome. Nucleic Acids Res. 2021;49:D134–43.
Zhou Y, Zeng P, Li YH, Zhang Z, Cui Q. SRAMP: prediction of mammalian N6-methyladenosine (m6A) sites based on sequence-derived features. Nucleic Acids Res. 2016;44:e91.
Chen K, Wei Z, Zhang Q, Wu X, Rong R, Lu Z, Su J, de Magalhaes JP, Rigden DJ, Meng J. WHISTLE: a high-accuracy map of the human N6-methyladenosine (m6A) epitranscriptome predicted using a machine learning approach. Nucleic Acids Res. 2019;47:e41.
Deng S, Zhang H, Zhu K, Li X, Ye Y, Li R, Liu X, Lin D, Zuo Z, Zheng J. M6A2Target: a comprehensive database for targets of m6A writers, erasers and readers. Brief Bioinformatics. 2021;22(3):bbaa055.
Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42:D92–7.
Zhang Q, Wang Z, Cheng X, Wu H. lncRNA DANCR promotes the migration an invasion and of trophoblast cells through microRNA-214-5p in preeclampsia. Bioengineered. 2021;12:9424–34.
Zhang Y, Yang H, Long Y, Zhang Y, Chen R, Shi J, Chen J. circRNA N6-methyladenosine methylation in preeclampsia and the potential role of N6-methyladenosine-modified circPAPPA2 in trophoblast invasion. Sci Rep. 2021;11:24357.
Fan W, Zhou W, Yan Q, Peng Y, Wang H, Kong C, Zhang B, Yu B, Chen L, Xue P. Upregulation of METTL14 contributes to trophoblast dysfunction by elevating FOXO3a expression in an m (6)A-dependent manner. Placenta. 2022;124:18–27.
Jacobo-Baca G, Salazar-Ybarra RA, Torres-de-la-Cruz V, Guzman-Lopez S, Elizondo-Omana RE, Guzman-Lopez A, Vazquez-Barragan MA, Martinez-de-Villarreal LE. Proteomic profile of preeclampsia in the first trimester of pregnancy. J Matern Fetal Neonatal Med. 2022;35:3446–52.
Gu Y, Chu X, Morgan JA, Lewis DF, Wang Y. Upregulation of METTL3 expression and m6A RNA methylation in placental trophoblasts in preeclampsia. Placenta. 2021;103:43–9.
Guo Y, Song W, Yang Y. Inhibition of ALKBH5-mediated m (6) A modification of PPARG mRNA alleviates H/R-induced oxidative stress and apoptosis in placenta trophoblast. Environ Toxicol. 2022;37:910–24.
Li XC, Jin F, Wang BY, Yin XJ, Hong W, Tian FJ. The m6A demethylase ALKBH5 controls trophoblast invasion at the maternal-fetal interface by regulating the stability of CYR61 mRNA. Theranostics. 2019;9:3853–65.
He J, Li X, Lu M, Wang J, Tang J, Luo S, Qian Y. ALKBH5 suppresses migration and invasion of human trophoblast cells by inhibiting epithelial-mesenchymal transition. Nan Fang Yi Ke Da Xue Xue Bao. 2020;40:1720–5.
Zheng Q, Yang F, Gan H, Jin L. Hypoxia induced ALKBH5 prevents spontaneous abortion by mediating m (6)A-demethylation of SMAD1/5 mRNAs. Biochim Biophys Acta Mol Cell Res. 2022;1869: 119316.
Klemetti M, Hiltunen LM, Heino S, Heinonen S, Kajantie E, Laivuori H. An obesity-related FTO variant and the risk of preeclampsia in a Finnish study population. J Pregnancy. 2011;2011:251470.
Guo L, Liu Y, Guo Y, Yang Y, Chen B. MicroRNA-423-5p inhibits the progression of trophoblast cells via targeting IGF2BP1. Placenta. 2018;74:1–8.
Wu L, Song WY, Xie Y, Hu LL, Hou XM, Wang R, Gao Y, Zhang JN, Zhang L, Li WW, Zhu C, Gao ZY, Sun YP. miR-181a-5p suppresses invasion and migration of HTR-8/SVneo cells by directly targeting IGF2BP2. Cell Death Dis. 2018;9:16.
Yang Q, Ma Y, Liu Y, Shao X, Jia W, Yu X, Li YX, Yang L, Gu W, Wang H, Wang J, Wang YL. MNSFbeta regulates placental development by conjugating IGF2BP2 to enhance trophoblast cell invasiveness. Cell Prolif. 2021;54:e13145.
Li H, Ouyang Y, Sadovsky E, Parks WT, Chu T, Sadovsky Y. Unique microRNA Signals in plasma exosomes from pregnancies complicated by preeclampsia. Hypertension. 2020;75:762–71.
Zhang H, He Y, Wang JX, Chen MH, Xu JJ, Jiang MH, Feng YL, Gu YF. miR-30-5p-mediated ferroptosis of trophoblasts is implicated in the pathogenesis of preeclampsia. Redox Biol. 2020;29:101402.
Lv Y, Lu C, Ji X, Miao Z, Long W, Ding H, Lv M. Roles of microRNAs in preeclampsia. J Cell Physiol. 2019;234:1052–61.
Sun N, Qin S, Zhang L, Liu S. Roles of noncoding RNAs in preeclampsia. Reprod Biol Endocrinol. 2021;19:100.
Chen A, Yu R, Jiang S, Xia Y, Chen Y. Recent advances of microRNAs, long non-coding RNAs, and circular RNAs in preeclampsia. Front Physiol. 2021;12:659638.
Wang D, Guan H, Xia Y. YTHDC1 maintains trophoblasts function by promoting degradation of m6A-modified circMPP1. Biochem Pharmacol. 2023;210:115456.
Li W, Liu D, Chang W, Lu X, Wang YL, Wang H, Zhu C, Lin HY, Zhang Y, Zhou J, Wang H. Role of IGF2BP3 in trophoblast cell invasion and migration. Cell Death Dis. 2014;5:e1025.
Shi H, Wang X, Lu Z, Zhao BS, Ma H, Hsu PJ, Liu C, He C. YTHDF3 facilitates translation and decay of N (6)-methyladenosine-modified RNA. Cell Res. 2017;27:315–28.
Chang G, Shi L, Ye Y, Shi H, Zeng L, Tiwary S, Huse JT, Huo L, Ma L, Ma Y, Zhang S, Zhu J, Xie V, Li P, Han L, He C, Huang S. YTHDF3 Induces the Translation of m (6)A-enriched gene transcripts to promote breast cancer brain metastasis. Cancer Cell. 2020;38:857–71.
Zheng Q, Gan H, Yang F, Yao Y, Hao F, Hong L, Jin L. Cytoplasmic m (1)A reader YTHDF3 inhibits trophoblast invasion by downregulation of m (1)A-methylated IGF1R. Cell Discovery. 2020;6:12.
Yang Y, Le Ray I, Zhu J, Zhang J, Hua J, Reilly M. Preeclampsia prevalence, risk factors, and pregnancy outcomes in Sweden and China. JAMA Netw Open. 2021;4:e218401.
Melchiorre K, Giorgione V, Thilaganathan B. The placenta and preeclampsia: villain or victim? Am J Obstet Gynecol. 2022;226:S954–62.
Bartsch E, Medcalf KE, Park AL, Ray JG. Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ. 2016;353:i1753.
Staff AC, Fjeldstad HE, Fosheim IK, Moe K, Turowski G, Johnsen GM, Alnaes-Katjavivi P, Sugulle M. Failure of physiological transformation and spiral artery atherosis: their roles in preeclampsia. Am J Obstet Gynecol. 2022;226:S895–906.
Chen J, Ning Y, Zhang H, Song N, Gu Y, Shi Y, Cai J, Ding X, Zhang X. METTL14-dependent m6A regulates vascular calcification induced by indoxyl sulfate. Life Sci. 2019;239:117034.
Mo XB, Lei SF, Zhang YH, Zhang H. Examination of the associations between m (6)A-associated single-nucleotide polymorphisms and blood pressure. Hypertens Res. 2019;42:1582–9.
Yang Y, Shen F, Huang W, Qin S, Huang JT, Sergi C, Yuan BF, Liu SM. Glucose is involved in the dynamic regulation of m6A in patients with type 2 diabetes. J Clin Endocrinol Metab. 2019;104:665–73.
Wang J, Wang K, Liu W, Cai Y, Jin H. m6A mRNA methylation regulates the development of gestational diabetes mellitus in Han Chinese women. Genomics. 2021;113:1048–56.
Du R, Bai Y, Li L. Biological networks in gestational diabetes mellitus: insights into the mechanism of crosstalk between long non-coding RNA and N (6)-methyladenine modification. BMC Pregnancy Childbirth. 2022;22:384.
Spradley FT. Metabolic abnormalities and obesity’s impact on the risk for developing preeclampsia. Am J Physiol Regul Integr Comp Physiol. 2017;312:R5–12.
Shen WB, Ni J, Yao R, Goetzinger KR, Harman C, Reece EA, Wang B, Yang P. Maternal obesity increases DNA methylation and decreases RNA methylation in the human placenta. Reprod Toxicol. 2022;107:90–6.
Bilbul M, Caccese C, Horsley K, Gauvreau A, Gavanski I, Montreuil T, Konci R, Lai JK, Da CD, Zelkowitz P, Shen HC, Gryte KR, Larosa A, Brown RN, Suarthana E, Nguyen TV. Maternal anxiety, depression and vascular function during pregnancy. J Psychosom Res. 2022;154:110722.
Wang Q, Pan M, Zhang T, Jiang Y, Zhao P, Liu X, Gao A, Yang L, Hou J. Fear stress during pregnancy affects placental m6a-modifying enzyme expression and epigenetic modification levels. Front Genet. 2022;13:927615.
Acknowledgements
We thank all authors for their contributions. All figures were created with BioRender.com
Author information
Authors and Affiliations
Contributions
All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Corresponding author
Ethics declarations
Ethical Approval
The study was granted an exemption from the local institutional review board.
Informed Consent
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, T., Jiang, Z., Yang, N. et al. N6‐methyladenosine (m6A) Modification in Preeclampsia. Reprod. Sci. 30, 3144–3152 (2023). https://doi.org/10.1007/s43032-023-01250-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-023-01250-8