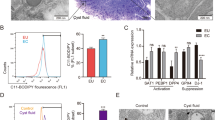
Abstract
Although nutrient status plays an important role in cell metabolism, its significance in endometriosis is obscure. Herein, we investigated the effects of a low-nutrient microenvironment on endometriosis. Stromal cells (SCs) from ovarian endometrioma (OESCs) or normal endometrium without endometriosis (NESCs) were isolated and cultured. A low-nutrient microenvironment was replicated by replacing the culture medium with Hank’s balanced salt solution. OESC and NESC proliferation under the low-nutrient condition was measured. The expression of exacerbating factors in endometriosis under the low-nutrient condition was examined at the mRNA and protein levels. OESCs showed higher proliferation than NESCs under the low-nutrient condition. In OESCs, the low-nutrient condition upregulated the mRNA expression of vascular endothelial growth factor (VEGF), interleukin-6 and -8, aromatase, Bcl-2, and peroxisome proliferator-activated receptor-gamma coactivator-1α (PGC-1α) and downregulated that of BAX and induced transcription of PI.3, PII, and exon II. Western blotting revealed elevated VEGF and PGC-1α expression under the low-nutrient condition in OESCs. These changes coincided with the elevated expression of PGC-1α, which was reduced at the mRNA level upon nutrient status rescue. Endometriosis is exacerbated by altered angiogenesis, inflammation, anti-apoptosis, and local estrogen production while trying to survive under a low-nutrient microenvironment; it may be attributed to PGC-1α-mediated metabolic mechanisms.





Similar content being viewed by others
Data Availability
The data underlying this article will be shared at reasonable request to the corresponding author.
Code Availability
Not applicable.
References
Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Viganò P. Endometriosis. Nat Rev Dis Primers. 2018;4(1):9.
Sampson JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol. 1927;3(2):93-110.43.
Qiu JJ, Liu MH, Zhang ZX, Chen LP, Yang QC, Liu HB. Transvaginal color Doppler sonography predicts ovarian interstitial fibrosis and microvascular injury in women with ovarian endometriotic cysts. Acta Obstet Gynecol Scand. 2012;91(5):605–12.
Püschel F, Favaro F, Redondo-Pedraza J, Lucendo E, Iurlaro R, Marchetti S, et al. Starvation and antimetabolic therapy promote cytokine release and recruitment of immune cells. Proc Natl Acad Sci U S A. 2020;117(18):9932–41.
Saunders PTK, Horne AW. Endometriosis: etiology, pathobiology, and therapeutic prospects. Cell. 2021;184(11):2807–24.
Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451(7181):1008–12.
Rius-Pérez S, Torres-Cuevas I, Millán I, Ortega ÁL, Pérez S. PGC-1α, Inflammation, and oxidative stress: an integrative view in metabolism. Oxid Med Cell Longev. 2020;2020:1452696.
Kataoka H, Mori T, Okimura H, Matsushima H, Ito F, Koshiba A, et al. Peroxisome proliferator-activated receptor-γ coactivator 1α-mediated pathway as a possible therapeutic target in endometriosis. Hum Reprod. 2019;34(6):1019–29.
Suganuma I, Mori T, Ito F, Tanaka Y, Sasaki A, Matsuo S, et al. Peroxisome proliferator-activated receptor gamma, coactivator 1α enhances local estrogen biosynthesis by stimulating aromatase activity in endometriosis. J Clin Endocrinol Metab. 2014;99(7):E1191–8.
Li C, Jung S, Lee S, Jeong D, Yang Y, Kim KI, et al. Nutrient/serum starvation derived TRIP-Br 3 down-regulation accelerates apoptosis by destabilizing XIAP. Oncotarget. 2015;6(10):7522–35.
Kondo A, Nonaka A, Shimamura T, Yamamoto S, Yoshida T, Kodama T, et al. Long noncoding RNA JHDM1D-AS1 promotes tumor growth by regulating angiogenesis in response to nutrient starvation. Mol Cell Biol. 2017;37(18):e00125-17.
Lin SC, Wang CC, Wu MH, Yang SH, Li YH, Tsai SJ. Hypoxia-induced microRNA-20a expression increases ERK phosphorylation and angiogenic gene expression in endometriotic stromal cells. J Clin Endocrinol Metab. 2012;97(8):E1515–23.
Yamanaka K, Xu B, Suganuma I, Kusuki I, Mita S, Shimizu Y, et al. Dienogest inhibits aromatase and cyclooxygenase-2 expression and prostaglandin E2 production in human endometriotic stromal cells in spheroid culture. Fertil Steril. 2012;97(2):477–82.
Manganelli V, Capozzi A, Recalchi S, Riitano G, Mattei V, Longo A, et al. The role of cardiolipin as a scaffold mitochondrial phospholipid in autophagosome formation: in vitro evidence. Biomolecules. 2021;11(2):222.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8.
Kinoshita Y, Chen S. Induction of aromatase (CYP19) expression in breast cancer cells through a nongenomic action of estrogen receptor alpha. Cancer Res. 2003;63(13):3546–55.
Mori T, Ito F, Matsushima H, Takaoka O, Koshiba A, Tanaka Y, et al. Dienogest reduces HSD17β1 expression and activity in endometriosis. J Endocrinol. 2015;225(2):69–76.
Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98(3):511–9.
McLaren J, Prentice A, Charnock-Jones DS, Smith SK. Vascular endothelial growth factor (VEGF) concentrations are elevated in peritoneal fluid of women with endometriosis. Hum Reprod. 1996;11(1):220–3.
Fasciani A, D’Ambrogio G, Bocci G, Monti M, Genazzani AR, Artini PG. High concentrations of the vascular endothelial growth factor and interleukin-8 in ovarian endometriomata. Mol Hum Reprod. 2000;6(1):50–4.
Laschke MW, Menger MD. Basic mechanisms of vascularization in endometriosis and their clinical implications. Hum Reprod Update. 2018;24(2):207–24.
Lu Z, Zhang W, Jiang S, Zou J, Li Y. Effect of oxygen tensions on the proliferation and angiogenesis of endometriosis heterograft in severe combined immunodeficiency mice. Fertil Steril. 2014;101(2):568–76.
Delbandi AA, Mahmoudi M, Shervin A, Akbari E, Jeddi-Tehrani M, Sankian M, et al. Eutopic and ectopic stromal cells from patients with endometriosis exhibit differential invasive, adhesive, and proliferative behavior. Fertil Steril. 2013;100(3):761–9.
Harada T, Yoshioka H, Yoshida S, Iwabe T, Onohara Y, Tanikawa M, et al. Increased interleukin-6 levels in peritoneal fluid of infertile patients with active endometriosis. Am J Obstet Gynecol. 1997;176(3):593–7.
Iwabe T, Harada T, Tsudo T, Tanikawa M, Onohara Y, Terakawa N. Pathogenetic significance of increased levels of interleukin-8 in the peritoneal fluid of patients with endometriosis. Fertil Steril. 1998;69(5):924–30.
Tseng JF, Ryan IP, Milam TD, Murai JT, Schriock ED, Landers DV, et al. Interleukin-6 secretion in vitro is up-regulated in ectopic and eutopic endometrial stromal cells from women with endometriosis. J Clin Endocrinol Metab. 1996;81(3):1118–22.
Li C, Zhao HL, Li YJ, Zhang YY, Liu HY, Feng FZ, et al. The expression and significance of leukemia inhibitory factor, interleukin-6 and vascular endothelial growth factor in Chinese patients with endometriosis. Arch Gynecol Obstet. 2021;304(1):163–70.
Velasco I, Rueda J, Acién P. Aromatase expression in endometriotic tissues and cell cultures of patients with endometriosis. Mol Hum Reprod. 2006;12(6):377–81.
Li MQ, Luo XZ, Meng YH, Mei J, Zhu XY, Jin LP, et al. CXCL8 enhances proliferation and growth and reduces apoptosis in endometrial stromal cells in an autocrine manner via a CXCR1-triggered PTEN/AKT signal pathway. Hum Reprod. 2012;27(7):2107–16.
Yoshino O, Izumi G, Shi J, Osuga Y, Hirota Y, Hirata T, et al. Activin-A is induced by interleukin-1β and tumor necrosis factor-α and enhances the mRNA expression of interleukin-6 and protease-activated receptor-2 and proliferation of stromal cells from endometrioma. Fertil Steril. 2011;96(1):118–21.
Steller H. Mechanisms and genes of cellular suicide. Science. 1995;267(5203):1445–9.
Agic A, Djalali S, Diedrich K, Hornung D. Apoptosis in endometriosis. Gynecol Obstet Invest. 2009;68(4):217–23.
Béliard A, Noël A, Foidart JM. Reduction of apoptosis and proliferation in endometriosis. Fertil Steril. 2004;82(1):80–5.
Taniguchi F, Kaponis A, Izawa M, Kiyama T, Deura I, Ito M, et al. Apoptosis and endometriosis. Front Biosci (Elite Ed). 2011;3:648–62.
Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Annu Rev Immunol. 1998;16:395–419.
Meresman GF, Vighi S, Buquet RA, Contreras-Ortiz O, Tesone M, Rumi LS. Apoptosis and expression of Bcl-2 and Bax in eutopic endometrium from women with endometriosis. Fertil Steril. 2000;74(4):760–6.
Bulun SE, Economos K, Miller D, Simpson ER. CYP19 (aromatase cytochrome P450) gene expression in human malignant endometrial tumors. J Clin Endocrinol Metab. 1994;79(6):1831–4.
Harada N. Aberrant expression of aromatase in breast cancer tissues. J Steroid Biochem Mol Biol. 1997;61(3–6):175–84.
Kitawaki J, Noguchi T, Amatsu T, Maeda K, Tsukamoto K, Yamamoto T, et al. Expression of aromatase cytochrome P450 protein and messenger ribonucleic acid in human endometriotic and adenomyotic tissues but not in normal endometrium. Biol Reprod. 1997;57(3):514–9.
Mori T, Ito F, Koshiba A, Kataoka H, Takaoka O, Okimura H, et al. Local estrogen formation and its regulation in endometriosis. Reprod Med Biol. 2019;18(4):305–11.
Babbar M, Huang Y, An J, Landas SK, Sheikh MS. CHTM1, a novel metabolic marker deregulated in human malignancies. Oncogene. 2018;37(15):2052–66.
Acknowledgements
We thank Ms. Ayaka Miura and Ms. Makoto Kazui for providing technical assistance.
Funding
This work was supported partially by Grants-in-Aid for Scientific Research under Grant number [21K09523] from the Ministry of Education, Culture, Sports, Science, and Technology (Japan).
Author information
Authors and Affiliations
Contributions
K.S., T.M., and J.K. designed the study. K.S., Y.T., M.F., K.O., E.M., Y.T., H.O., H.K., O.T., and Y.T. conceived and carried out experiments. K.S., T.M., F.I., A.K., K.K., and I.K. contributed to the interpretation of the results. K.S. and T.M. wrote the article. All authors were involved in writing the paper and had final approval of the submitted and published version.
Corresponding author
Ethics declarations
Ethics Approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments. This study was approved by the institutional review board of the Kyoto Prefectural University of Medicine (ERB-C-855–1).
Consent to Participate
Informed consent was obtained from all patients for being included in the study.
Consent for Publication
All agreed to publish this article.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

Supplementary file2
Supplementary Fig. 1 TUNEL staining of ovarian endometrioma stromal cell (OESC) and normal endometrium stromal cell (NESC) under low-nutrient conditions. TUNEL staining was performed after 24 h of incubation in low-nutrient medium, respectively. a, NESCs; b, OESCs; arrows, positive cells; bar, 200 μm. (PNG 2930 kb)

Supplementary file3
Supplementary Fig. 2 Expression of IL-1β and TNF-α in ovarian endometrioma stromal cell (OESC) under low-nutrient conditions. The expression of them in OESCs (n=6) was measured using real-time polymerase chain reaction (PCR) after incubation with control (Cont) or low-nutrient (Low) medium for 24 h. *P < 0.05 versus Cont (Wilcoxon signed-rank test). Abbreviations: TNF-α, tumor necrosis factor-α (PNG 112 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shimura, K., Tarumi, Y., Fujii, M. et al. Low-Nutrient Environment-Induced Changes in Inflammation, Cell Proliferation, and PGC-1α Expression in Stromal Cells with Ovarian Endometriosis. Reprod. Sci. 30, 1094–1102 (2023). https://doi.org/10.1007/s43032-022-01089-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-022-01089-5