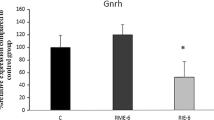
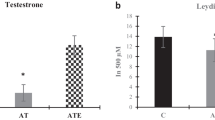
Abstract
The purpose of this study is to investigate the effect of aerobic exercise (AE) training and/or oyster peptide (OP) supplementation on the formation of late-onset hypogonadism (LOH). AE training and/or OP supplement was performed during Cytoxan (CTX)-induced LOH formation in male SD rats for 6 consecutive weeks. Low dose of CTX could decrease mating times, the levels of luteinizing hormone (LH), total testosterone (TT), free testosterone (FT) in serum and TT, androgen receptor (AR), androgen binding protein (ABP), and glutathione peroxidase (GSH-Px) in testicle, but increase capture latency, mating latency, and malondialdehyde, and downregulate the mRNA expression of steroidogenic acute regulatory (StAR), P450 cholesterol side chain cleavage enzyme (P450scc), and StAR-related lipid transfer domain 7 (StARD7) in testicle. Every change was altered by AE training combined with OP supplement significantly, except for serum LH. Moreover, the effect of AE training combined with OP supplement was better than that of AE training on serum TT, FSH, testicular TT, mating latency, capture times, and mating times. AE training combined with OP supplement during CTX-induced LOH formation can prevent the LOH development by enhancing pituitary-gonads axis’s function and reducing testicular oxidative stress to promote testosterone synthesis and spermatogenesis.
Graphical Abstract









Similar content being viewed by others

References
Huhtaniemi IT. Andropause–lessons from the European Male Ageing Study. Ann Endocrinol (Paris). 2014;75:128–31. https://doi.org/10.1016/j.ando.2014.03.005.
Isidori AM, Giannetta E, Gianfrilli D, Greco EA, Bonifacio V, Aversa A, Isidori A, Fabbri A, Lenzi A. Effects of testosterone on sexual function in men: results of a meta-analysis. Clin Endocrinol (Oxf). 2005;63:381–94. https://doi.org/10.1111/j.1365-2265.2005.02350.x.
Almehmadi Y, Yassin DJ, Yassin AA. Erectile dysfunction is a prognostic indicator of comorbidities in men with late onset hypogonadism. Aging Male. 2015;18:186–94. https://doi.org/10.3109/13685538.2015.1046044.
Rastrelli G, Corona G, Tarocchi M, Mannucci E, Maggi M. How to define hypogonadism? Results from a population of men consulting for sexual dysfunction. J Endocrinol Invest. 2016;39:473–84. https://doi.org/10.1007/s40618-015-0425-1.
Lunenfeld B, Mskhalaya G, Zitzmann M, Arver S, Kalinchenko S, Tishova Y, Morgentaler A. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male. 2015;18:5–15. https://doi.org/10.3109/13685538.2015.1004049.
Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589–98. https://doi.org/10.1210/jcem.87.2.8201.
Omar YA, Younis SE, Ismail IY, El-Sakka AI. Testosterone level and endothelial dysfunction in patients with vasculogenic erectile dysfunction. Andrology. 2017;5:527–34. https://doi.org/10.1111/andr.12347.
Corona G, Rastrelli G, Maggi M. Diagnosis and treatment of late-onset hypogonadism: systematic review and meta-analysis of TRT outcomes. Best Pract Res Clin Endocrinol Metab. 2013;27:557–79. https://doi.org/10.1016/j.beem.2013.05.002.
Choi SW, Jeon SH, Kwon EB, Zhu GQ, Lee KW, Choi JB, Jeong HC, Kim KS, Bae SR, Bae WJ, Kim SJ, Cho HJ, Ha US, Hong SH, Hwang SY, Kim SW. Effect of Korean herbal formula (modified Ojayeonjonghwan) on androgen receptor expression in an aging rat model of late onset hypogonadism. World J Mens Health. 2019;37:105–12. https://doi.org/10.5534/wjmh.180051.
Mazzeo RS. Aging, immune function, and exercise: hormonal regulation. Int J Sports Med. 2000;21 Suppl 1:S10–3. https://doi.org/10.1055/s-2000-1445.
Matos B, Howl J, Ferreira R, Fardilha M. Exploring the effect of exercise training on testicular function. Eur J Appl Physiol. 2019;119:1–8. https://doi.org/10.1007/s00421-018-3989-6.
Kumagai H, Yoshikawa T, Zempo-Miyaki A, Myoenzono K, Tsujimoto T, Tanaka K, Maeda S. Vigorous physical activity is associated with regular aerobic exercise-induced increased serum testosterone levels in overweight/obese men. Horm Metab Res. 2018;50:73–9. https://doi.org/10.1055/s-0043-117497.
Hayes LD, Herbert P, Sculthorpe NF, Grace FM. Exercise training improves free testosterone in lifelong sedentary aging men. Endocr Connect. 2017;6:306–10. https://doi.org/10.1530/EC-17-0082.
Seo DY, Lee SR, Kwak HB, Park H, Seo KW, Noh YH, Song KM, Ryu JK, Ko KS, Rhee BD, Han J. Exercise training causes a partial improvement through increasing testosterone and eNOS for erectile function in middle-aged rats. Exp Gerontol. 2018;108:131–8. https://doi.org/10.1016/j.exger.2018.04.003.
Scharhag J, Herrmann M, Urhausen A, Haschke M, Herrmann W, Kindermann W. Independent elevations of N-terminal pro-brain natriuretic peptide and cardiac troponins in endurance athletes after prolonged strenuous exercise. Am Heart J. 2005;150:1128–34. https://doi.org/10.1016/j.ahj.2005.01.051.
Jin QG, Shi WT, Wang YC, Li SY, Xue C, Xu HR, Wu MT, Wei Y. Oyster peptide prevents the occurrence of exercise-hypogonadal male condition by improving the function of pituitary gonadal axis in male rats. Andrologia. 2021;53:e14005. https://doi.org/10.1111/and.14005.
Yi X, Tang D, Cao S, Li T, Gao H, Ma T, Yao T, Li J, Chang B. Effect of different exercise loads on testicular oxidative stress and reproductive function in obese male mice. Oxid Med Cell Longev. 2020;2020:3071658. https://doi.org/10.1155/2020/3071658.
Hajizadeh MB, Tartibian B. Combined aerobic and resistance exercise training for improving reproductive function in infertile men: a randomized controlled trial. Appl Physiol Nutr Metab. 2017;42:1293–306. https://doi.org/10.1139/apnm-2017-0249.
Bahadorani M, Tavalaee M, Abedpoor N, Ghaedi K, Nazem MN, Nasr-Esfahani MH. Effects of branched-chain amino acid supplementation and/or aerobic exercise on mouse sperm quality and testosterone production. Andrologia. 2019;51:e13183. https://doi.org/10.1111/and.13183.
Schwarz ER, Willix RJ. Impact of a physician-supervised exercise-nutrition program with testosterone substitution in partial androgen-deficient middle-aged obese men. J Geriatr Cardiol. 2011;8:201–6. https://doi.org/10.3724/SP.J.1263.2011.00201.
Chen H, Cheng S, Fan F, Tu M, Xu Z, Du M. Identification and molecular mechanism of antithrombotic peptides from oyster proteins released in simulated gastro-intestinal digestion. Food Funct. 2019;10:5426–35. https://doi.org/10.1039/c9fo01433k.
Cheng S, Tu M, Chen H, Xu Z, Wang Z, Liu H, Zhao G, Zhu B, Du M. Identification and inhibitory activity against alpha-thrombin of a novel anticoagulant peptide derived from oyster (Crassostrea gigas) protein. Food Funct. 2018;9:6391–400. https://doi.org/10.1039/c8fo01635f.
Wang J, Hu J, Cui J, Bai X, Du Y, Miyaguchi Y, Lin B. Purification and identification of a ACE inhibitory peptide from oyster proteins hydrolysate and the antihypertensive effect of hydrolysate in spontaneously hypertensive rats. Food Chem. 2008;111:302–8. https://doi.org/10.1016/j.foodchem.2008.03.059.
Wang YK, He HL, Wang GF, Wu H, Zhou BC, Chen XL, Zhang YZ. Oyster (Crassostrea gigas) hydrolysates produced on a plant scale have antitumor activity and immunostimulating effects in BALB/c mice. Mar Drugs. 2010;8:255–68. https://doi.org/10.3390/md8020255.
Feng C, Tian L, Jiao Y, Tan Y, Liu C, Luo Y, Hong H. The effect of steam cooking on the proteolysis of pacific oyster (Crassostrea gigas) proteins: digestibility, allergenicity, and bioactivity. Food Chem. 2022;379:132160. https://doi.org/10.1016/j.foodchem.2022.132160.
Jin Q, Zhou M, Hu Y, Chen L, Liu Y, Wang Y, Wu M, Zhang R, Liu W. Inhibitory effect and mechanism of oyster enzymatic hydrolysate on lung metastasis in the subcutaneous Lewis lung cancer model in mice. Kafkas Univ Vet Fak. 2020. https://doi.org/10.9775/kvfd.2020.24776.
Moon PD, Kim MH, Lim HS, Oh HA, Nam SY, Han NR, Kim MJ, Jeong HJ, Kim HM. Taurine, a major amino acid of oyster, enhances linear bone growth in a mouse model of protein malnutrition. BioFactors. 2015;41:190–7. https://doi.org/10.1002/biof.1213.
Li M, Zhou M, Wei Y, Jia F, Yan Y, Zhang R, Cai M, Gu R. The beneficial effect of oyster peptides and oyster powder on cyclophosphamide-induced reproductive impairment in male rats: a comparative study. J Food Biochem. 2020;44:e13468. https://doi.org/10.1111/jfbc.13468.
Jin Q, Ma Y, Shi W, Wang J, Zhao R, Zhang H, Wu M, Liu W. Oyster oligopeptide improving cyclophosphamide-induced partial androgen deficiency of the aging male by promotion of testosterone synthesis. Geriatr Gerontol Int. 2021. https://doi.org/10.1111/ggi.14129.
He QH, Zhou X. Study of partial and rogen deficiency model induced by cyclophosphamide in rats. J Hunan Univ Tradit Chin Med. 2011;31:15–7+29.
Jeong HC, Jeon SH, Guan QZ, Bashraheel F, Choi SW, Kim SJ, Bae WJ, Cho HJ, Ha US, Hong SH, Lee JY, Hong SB, Kim SW. Lycium chinense mill improves hypogonadism via anti-oxidative stress and anti-apoptotic effect in old aged rat model. Aging Male. 2020;23:287–96. https://doi.org/10.1080/13685538.2018.1498079.
Qu M, Zhao Y, Qing X, Zhang X, Li H. Androgen-dependent miR-125a-5p targets LYPLA1 and regulates global protein palmitoylation level in late-onset hypogonadism males. J Cell Physiol. 2021;236:4738–49. https://doi.org/10.1002/jcp.30195.
Li WR, Chen L, Chang ZJ, Xin H, Liu T, Zhang YQ, Li GY, Zhou F, Gong YQ, Gao ZZ, Xin ZC. Autophagic deficiency is related to steroidogenic decline in aged rat Leydig cells. Asian J Androl. 2011;13:881–8. https://doi.org/10.1038/aja.2011.85.
Nutsch VL, Will RG, Tobiansky DJ, Reilly MP, Gore AC, Dominguez JM. Age-related changes in sexual function and steroid-hormone receptors in the medial preoptic area of male rats. Horm Behav. 2017;96:4–12. https://doi.org/10.1016/j.yhbeh.2017.08.008.
Alshinnawy AS, El-Sayed WM, Taha AM, Sayed AA, Salem AM. Astragalus membranaceus and Punica granatum alleviate infertility and kidney dysfunction induced by aging in male rats. Turk J Biol. 2020;44:166–75. https://doi.org/10.3906/biy-2001-5.
Chen ZL, Zhang XC, Xue Q, Yang Q. Effect of cyclophosphamide on mouse spermatogenic obstacles. Prog Vet Med. 2017;38:34–8. https://doi.org/10.16437/j.cnki.1007-5038.2017.05.008.
Chen Q, Shan C, Su J, Chen W, Zhu J, Chen S, Lyu G. Effect of Wubi Shanyao pills on sexual function in mice with kidney-yang-deficiency induced by hydrocortisone. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49:697–704. https://doi.org/10.3785/j.issn.1008-9292.2020.12.04.
Afifi M, Almaghrabi OA, Kadasa NM. Ameliorative effect of zinc oxide nanoparticles on antioxidants and sperm characteristics in streptozotocin-induced diabetic rat testes. Biomed Res Int. 2015;2015:153573. https://doi.org/10.1155/2015/153573.
Cosentino MJ, Nishida M, Rabinowitz R, Cockett AT. Histopathology of prepubertal rat testes subjected to various durations of spermatic cord torsion. J Androl. 1986;7:23–31. https://doi.org/10.1002/j.1939-4640.1986.tb00862.x.
Erdem AO, Coskun OD, Baser AT, Sirinyildiz F, Ek R, Culhaci N, Yazici M, Ozkisacik S. Comparison of the effects of intermittent reperfusion and hypothermia in preventing testicular ischemia-reperfusion injury in the testicular torsion model in rats. J Pediatr Urol. 2019;15:617–23. https://doi.org/10.1016/j.jpurol.2019.09.013.
Wang C, Nieschlag E, Swerdloff R, Behre HM, Hellstrom WJ, Gooren LJ, Kaufman JM, Legros JJ, Lunenfeld B, Morales A, Morley JE, Schulman C, Thompson IM, Weidner W, Wu FC. ISA, ISSAM, EAU, EAA and ASA recommendations: investigation, treatment and monitoring of late-onset hypogonadism in males. Int J Impot Res. 2009;21:1–8. https://doi.org/10.1038/ijir.2008.41.
Chen Y, Li L, Yan CY, Wang ZS, Sui WK, Yang P, Wang HF, Li RX, Li YK, Lian FL, Li D, Feng QL, Feng ZJ, Zeng XY, Meng FY, Bu GX, Cao XH, Du XG. Effects of small molecule polypeptide of oyster on sexual function in male mice. Genomics Appl Biol. 2019;38(1):109–16. https://doi.org/10.13417/j.gab.038.000109.
Gill-Sharma MK. Testosterone retention mechanism in Sertoli cells: a biochemical perspective. Open Biochem J. 2018;12:103–12. https://doi.org/10.2174/1874091X01812010103.
Manna PR, Stetson CL, Slominski AT, Pruitt K. Role of the steroidogenic acute regulatory protein in health and disease. Endocrine. 2016;51:7–21. https://doi.org/10.1007/s12020-015-0715-6.
Zirkin BR, Papadopoulos V. Leydig cells: formation, function, and regulation. Biol Reprod. 2018;99:101–11. https://doi.org/10.1093/biolre/ioy059.
Stocco DM, Zhao AH, Tu LN, Morohaku K, Selvaraj V. A brief history of the search for the protein(s) involved in the acute regulation of steroidogenesis. Mol Cell Endocrinol. 2017;441:7–16. https://doi.org/10.1016/j.mce.2016.07.036.
Chen H, Hardy MP, Zirkin BR. Age-related decreases in Leydig cell testosterone production are not restored by exposure to LH in vitro. Endocrinology. 2002;143:1637–42. https://doi.org/10.1210/endo.143.5.8802.
Beattie MC, Adekola L, Papadopoulos V, Chen H, Zirkin BR. Leydig cell aging and hypogonadism. Exp Gerontol. 2015;68:87–91. https://doi.org/10.1016/j.exger.2015.02.014.
Das UB, Mallick M, Debnath JM, Ghosh D. Protective effect of ascorbic acid on cyclophosphamide- induced testicular gametogenic and androgenic disorders in male rats. Asian J Androl. 2002;4:201–7.
Huang D, Wei W, Xie F, Zhu X, Zheng L, Lv Z. Steroidogenesis decline accompanied with reduced antioxidation and endoplasmic reticulum stress in mice testes during ageing. Andrologia. 2018;50. https://doi.org/10.1111/and.12816.
Zhao YT, Qi YW, Hu CY, Chen SH, Liu Y. Advanced glycation end products inhibit testosterone secretion by rat Leydig cells by inducing oxidative stress and endoplasmic reticulum stress. Int J Mol Med. 2016;38:659–65. https://doi.org/10.3892/ijmm.2016.2645.
Diemer T, Allen JA, Hales KH, Hales DB. Reactive oxygen disrupts mitochondria in MA-10 tumor Leydig cells and inhibits steroidogenic acute regulatory (StAR) protein and steroidogenesis. Endocrinology. 2003;144:2882–91. https://doi.org/10.1210/en.2002-0090.
Jana K, Jana N, De DK, Guha SK. Ethanol induces mouse spermatogenic cell apoptosis in vivo through over-expression of Fas/Fas-L, p53, and caspase-3 along with cytochrome c translocation and glutathione depletion. Mol Reprod Dev. 2010;77:820–33. https://doi.org/10.1002/mrd.21227.
Jana K, Samanta PK, De DK. Nicotine diminishes testicular gametogenesis, steroidogenesis, and steroidogenic acute regulatory protein expression in adult albino rats: possible influence on pituitary gonadotropins and alteration of testicular antioxidant status. Toxicol Sci. 2010;116:647–59. https://doi.org/10.1093/toxsci/kfq149.
Hajizadeh MB, Tartibian B. Moderate aerobic exercise training for improving reproductive function in infertile patients: a randomized controlled trial. Cytokine. 2017;92:55–67. https://doi.org/10.1016/j.cyto.2017.01.007.
Zhao X, Bian Y, Sun Y, Li L, Wang L, Zhao C, Shen Y, Song Q, Qu Y, Niu S, Wu W, Gao F. Effects of moderate exercise over different phases on age-related physiological dysfunction in testes of SAMP8 mice. Exp Gerontol. 2013;48:869–80. https://doi.org/10.1016/j.exger.2013.05.063.
Zhu Q, Cui YG. Research progress of regulatory mechanism of spermatogenesis. J Reprod Med. 2016;25:378–83.
Jana K, Yin X, Schiffer RB, Chen JJ, Pandey AK, Stocco DM, Grammas P, Wang X. Chrysin, a natural flavonoid enhances steroidogenesis and steroidogenic acute regulatory protein gene expression in mouse Leydig cells. J Endocrinol. 2008;197:315–23. https://doi.org/10.1677/JOE-07-0282.
Blanco-Rodriguez J, Martinez-Garcia C. Apoptosis precedes detachment of germ cells from the seminiferous epithelium after hormone suppression by short-term oestradiol treatment of rats. Int J Androl. 1998;21:109–15. https://doi.org/10.1046/j.1365-2605.1998.00109.x.
Funding
This work was supported by National Key Research and Development project in China, Grant/Award Number: 2016YFD0400603, and Open Project of the Beijing Engineering Research Center of Protein and Functional Peptides, Grant/Award Number: 2017PFP002.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Wenting Shi, Yu Liu, Qiguan Jin, Meitong Wu, Qizheng Sun, Zheng Li, and Wenying Liu. The first draft of the manuscript was written by Wenting Shi and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
The study was conducted according to the guidelines of the Declaration of Helsinki. The experimental procedures were approved by the Experimental Animal Management Committee and Experimental Animal Ethics Committee of Yangzhou University (YzuDWLL-201804–001).
Conflict of Interest
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, W., Liu, Y., Jin, Q. et al. Effects of Aerobic Exercise Combined with Oyster Peptide Supplement on the Formation of CTX-induced Late-Onset Hypogonadism in Male Rats. Reprod. Sci. 30, 1291–1305 (2023). https://doi.org/10.1007/s43032-022-01068-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-022-01068-w