Abstract
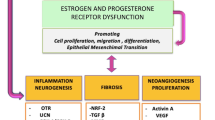
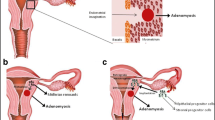
Adenomyosis is an estrogen-dependent gynecologic disease characterized by the presence of endometrial tissue within the myometrium. Adenomyosis presents with abnormal uterine bleeding, pelvic pains, and infertility. This review aimed to investigate the major estrogen downstream effectors involved in the process of adenomyosis development and their potential use for nonhormonal treatment. A literature search was performed for preclinical and clinical studies published between January 2010 and November 2021 in the PubMed and Google Scholar databases using a combination of specific terms. Adenomyosis presents with a wide spectrum of clinical manifestations from asymptomatic to severe through a complex process involving a series of molecular changes associated with inflammation, invasion, angiogenesis, and fibrosis. Adenomyosis may develop through a multistep process, including the acquisition of (epi)genetic mutations, tissue injury caused at the endometrial–myometrial interface, inside-to-outside invasion (from the endometrial side into the uterine wall), or outside-to-inside invasion (from the serosal side into the uterine wall), and epithelial–mesenchymal transition, tissue repair or remodeling in the myometrium. These processes can be regulated by increased estrogen biosynthesis and progesterone resistance. The expression of estrogen downstream effectors associated with persistent inflammation, fragile and more permeable vessel formation, and tissue injury and remodeling may be correlated with dysmenorrhea, heavy menstrual bleeding, and infertility, respectively. Key estrogen downstream targets (e.g., WNT/β-catenin, transforming growth factor-β, and nuclear factor-κB) may serve as hub genes. We reviewed the molecular mechanisms underlying the development of adenomyosis and summarized potential nonhormonal therapies.




Similar content being viewed by others
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author.
References
Senturk LM, Imamoglu M. Adenomyosis: what is new? Womens Health (Lond). 2015;11(5):717–24.
Lacheta J. Uterine adenomyosis: pathogenesis, diagnostics, symptomatology and treatment. Ceska Gynekol. 2019;84(3):240–6.
Harmsen MJ, Wong CFC, Mijatovic V, Griffioen AW, Groenman F, Hehenkamp WJK, et al. Role of angiogenesis in adenomyosis-associated abnormal uterine bleeding and subfertility: a systematic review. Hum Reprod Update. 2019;25(5):647–71.
Xiaoyu L, Weiyuan Z, Ping J, Anxia W, Liane Z. Serum differential proteomic analysis of endometriosis and adenomyosis by iTRAQ technique. Eur J Obstet Gynecol Reprod Biol. 2014;182:62–5.
Maruyama S, Imanaka S, Nagayasu M, Kimura M, Kobayashi H. Relationship between adenomyosis and endometriosis; different phenotypes of a single disease? Eur J Obstet Gynecol Reprod Biol. 2020;253:191–7.
Bulun SE, Yildiz S, Adli M, Wei JJ. Adenomyosis pathogenesis: insights from next-generation sequencing. Hum Reprod Update. 2021;27(6):1086–97.
Kobayashi H, Matsubara S, Imanaka S. Relationship between magnetic resonance imaging-based classification of adenomyosis and disease severity. J Obstet Gynaecol Res. 2021;47(7):2251–60.
Leyendecker G, Wildt L, Mall G. The pathophysiology of endometriosis and adenomyosis: tissue injury and repair. Arch Gynecol Obstet. 2009;280(4):529–38.
Leyendecker G, Wildt L. A new concept of endometriosis and adenomyosis: tissue injury and repair (TIAR). Horm Mol Biol Clin Investig. 2011;5(2):125–42.
Benagiano G, Brosens I, Habiba M. Structural and molecular features of the endomyometrium in endometriosis and adenomyosis. Hum Reprod Update. 2014;20(3):386–402.
Benagiano G, Brosens I. The endometrium in adenomyosis. Womens Health (Lond). 2012;8(3):301–12.
Kobayashi H, Kishi Y, Matsubara S. Mechanisms underlying adenomyosis-related fibrogenesis. Gynecol Obstet Invest. 2020;85(1):1–12.
Bourdon M, Santulli P, Jeljeli M, Vannuccini S, Marcellin L, Doridot L, et al. Immunological changes associated with adenomyosis: a systematic review. Hum Reprod Update. 2021;27(1):108–29.
Bulun SE, Gurates B, Fang Z, Tamura M, Sebastian S, Zhou J, et al. Mechanisms of excessive estrogen formation in endometriosis. J Reprod Immunol. 2002;55(1):21–33.
Zhai J, Vannuccini S, Petraglia F, Giudice LC. Adenomyosis: mechanisms and pathogenesis. Semin Reprod Med. 2020;38(2–03):129–43.
Inoue S, Hirota Y, Ueno T, Fukui Y, Yoshida E, Hayashi T, et al. Uterine adenomyosis is an oligoclonal disorder associated with KRAS mutations. Nat Commun. 2019;10(1):5785.
Mullen J, Kato S, Sicklick JK, Kurzrock R. Targeting ARID1A mutations in cancer. Cancer Treat Rev. 2021;100: 102287.
Reske JJ, Wilson MR, Holladay J, Wegener M, Adams M, Chandler RL. SWI/SNF inactivation in the endometrial epithelium leads to loss of epithelial integrity. Hum Mol Genet. 2020;29(20):3412–30.
Oie S, Matsuzaki K, Yokoyama W, Murayama A, Yanagisawa J. HDAC3 regulates stability of estrogen receptor alpha mRNA. Biochem Biophys Res Commun. 2013;432(2):236–41.
Liu X, Nie J, Guo SW. Elevated immunoreactivity against class I histone deacetylases in adenomyosis. Gynecol Obstet Invest. 2012;74(1):50–5.
Liu Xishi, Yuan Lei, Guo SW. Valproic acid as a therapy for adenomyosis: a comparative case series. Reprod Sci. 2010;17(10):904–12.
Campo S, Campo V, Benagiano G. Adenomyosis and infertility. Reprod Biomed Online. 2012;24(1):35–46.
Li T, Li YG, Pu DM. Matrix metalloproteinase-2 and -9 expression correlated with angiogenesis in human adenomyosis. Gynecol Obstet Invest. 2006;62(4):229–35.
Yi KW, Kim SH, Ihm HJ, Oh YS, Chae HD, Kim CH, Kang BM. Increased expression of p21-activated kinase 4 in adenomyosis and its regulation of matrix metalloproteinase-2 and -9 in endometrial cells. Fertil Steril. 2015;103(4):1089-1097.e2.
Mori T, Yamasaki S, Masui F, Matsuda M, Sasabe H, Zhou YF. Suppression of the development of experimentally induced uterine adenomyosis by a novel matrix metalloproteinase inhibitor, ONO-4817, in mice. Exp Biol Med (Maywood). 2001;226(5):429–33.
DiDonato JA, Mercurio F, Karin M. NF-kappaB and the link between inflammation and cancer. Immunol Rev. 2012;246(1):379–400.
Park H, Kim SH, Cho YM, Ihm HJ, Oh YS, Hong SH, et al. Increased expression of nuclear factor kappa-B p65 subunit in adenomyosis. Obstet Gynecol Sci. 2016;59(2):123–9.
Yu J, Chen LH, Zhang B, Zheng QM. The modulation of endometriosis by lncRNA MALAT1 via NF-kappaB/iNOS. Eur Rev Med Pharmacol Sci. 2019;23(10):4073–80.
Oh SJ, Shin JH, Kim TH, Lee HS, Yoo JY, Ahn JY, et al. β-Catenin activation contributes to the pathogenesis of adenomyosis through epithelial-mesenchymal transition. J Pathol. 2013;231(2):210–22.
Klemmt PAB, Starzinski-Powitz A. Molecular and Cellular Pathogenesis of Endometriosis. Curr Womens Health Rev. 2018;14(2):106–16.
Yang JH, Chen MJ, Wu MY, Chen YC, Yang YS, Ho HN. Decreased suppression of interleukin-6 after treatment with medroxyprogesterone acetate and danazol in endometrial stromal cells of women with adenomyosis. Fertil Steril. 2006;86(5):1459–65.
Wang Q, Wang L, Shao J, Wang Y, Jin LP, Li DJ, et al. L-22 enhances the invasiveness of endometrial stromal cells of adenomyosis in an autocrine manner. Int J Clin Exp Pathol. 2014;7(9):5762–71.
Sotnikova N, Antsiferova I, Malyshkina A. Cytokine network of eutopic and ectopic endometrium in women with adenomyosis. Am J Reprod Immunol. 2002;47(4):251–5.
Carrarelli P, Yen CF, Funghi L, Arcuri F, Tosti C, Bifulco G, et al. Expression of inflammatory and neurogenic mediators in adenomyosis. Reprod Sci. 2017;24(3):369–75.
Makrigiannakis A, Vrekoussis T, Zoumakis E, Hatzidakis V, Vlachou E, Salakos N, et al. Endometrial CRH and implantation: from bench to bedside. Hormones (Athens). 2018;17(3):293–7.
AlAshqar A, Reschke L, Kirschen GW, Borahay MA. Role of inflammation in benign gynecologic disorders: from pathogenesis to novel therapies. Biol Reprod. 2021;105(1):7–31.
Prašnikar E, Kunej T, Knez J, Repnik K, Potočnik U, Kovačič B. Determining the molecular background of endometrial receptivity in adenomyosis. Biomolecules. 2020;10(9):1311.
Kay N, Huang CY, Shiu LY, Yu YC, Chang Y, Schatz F, et al. TGF-beta1 neutralization improves pregnancy outcomes by restoring endometrial receptivity in mice with adenomyosis. Reprod Sci. 2021;28(3):877–87.
Zhou Y, Peng Y, Xia Q, Yan D, Zhang H, Zhang L, et al. Decreased Indian hedgehog signaling activates autophagy in endometriosis and adenomyosis. Reproduction. 2021;161(2):99–109.
van den Berg YW, van den Hengel LG, Myers HR, Ayachi O, Jordanova E, Ruf W, et al. Alternatively spliced tissue factor induces angiogenesis through integrin ligation. Proc Natl Acad Sci U S A. 2009;106(46):19497–502.
Shang WQ, Yu JJ, Zhu L, Zhou WJ, Chang KK, Wang Q, et al. Blocking IL-22, a potential treatment strategy for adenomyosis by inhibiting crosstalk between vascular endothelial and endometrial stromal cells. Am J Transl Res. 2015;7(10):1782–97.
Exacoustos C, Morosetti G, Conway F, Camilli S, Martire FG, Lazzeri L, et al. New sonographic classification of adenomyosis: do type and degree of adenomyosis correlate to severity of symptoms? J Minim Invasive Gynecol. 2020;27(6):1308–15.
Zhou S, Yi T, Liu R, Bian C, Qi X, He X, et al. Proteomics identification of annexin A2 as a key mediator in the metastasis and proangiogenesis of endometrial cells in human adenomyosis. Mol Cell Proteomics. 2012;11(7):M112.017988.
Liu X, Nie J, Guo SW. Elevated immunoreactivity to tissue factor and its association with dysmenorrhea severity and the amount of menses in adenomyosis. Hum Reprod. 2011;26(2):337–45.
Li B, Chen M, Liu X, Guo SW. Constitutive and tumor necrosis factor-alpha-induced activation of nuclear factor-kappaB in adenomyosis and its inhibition by andrographolide. Fertil Steril. 2013;100(2):568–77.
Ferenczy A. Pathophysiology of adenomyosis. Hum Reprod Update. 1998;4(4):312–22.
Smith OP, Jabbour HN, Critchley HO. Cyclooxygenase enzyme expression and E series prostaglandin receptor signalling are enhanced in heavy menstruation. Hum Reprod. 2007;22(5):1450–6.
García-Solares J, Donnez J, Donnez O, Dolmans MM. Pathogenesis of uterine adenomyosis: invagination or metaplasia? Fertil Steril. 2018;109(3):371–9.
Shen M, Liu X, Zhang H, Guo SW. Transforming growth factor beta1 signaling coincides with epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the development of adenomyosis in mice. Hum Reprod. 2016;31(2):355–69.
Yoo JY, Ku BJ, Kim TH, Il Ahn J, Ahn JY, Yang WS, Lim JM, Taketo MM, Shin JH, Jeong JW. beta-catenin activates TGF-beta-induced epithelial-mesenchymal transition in adenomyosis. Exp Mol Med. 2020;52(10):1754–65.
Wang T, Yuan J, Zhang J, Tian R, Ji W, Zhou Y, et al. Anxa2 binds to STAT3 and promotes epithelial to mesenchymal transition in breast cancer cells. Oncotarget. 2015;6(31):30975–92.
Kadota T, Fujita Y, Araya J, Watanabe N, Fujimoto S, Kawamoto H, et al. Human bronchial epithelial cell-derived extracellular vesicle therapy for pulmonary fibrosis via inhibition of TGF-beta-WNT crosstalk. J Extracell Vesicles. 2021;10(10):e12124.
An M, Li D, Yuan M, Li Q, Zhang L, Wang G. Different macrophages equally induce EMT in endometria of adenomyosis and normal. Reproduction. 2017;154(1):79–92.
An M, Li D, Yuan M, Li Q, Zhang L, Wang G. Interaction of macrophages and endometrial cells induces epithelial-mesenchymal transition-like processes in adenomyosis. Biol Reprod. 2017;96(1):46–57.
Maia H Jr, Haddad C, Pinheiro N, Casoy J. The effect of oral contraceptives on aromatase and Cox-2 expression in the endometrium of patients with idiopathic menorrhagia or adenomyosis. Int J Womens Health. 2013;5:293–9.
Maia H Jr, Haddad C, Casoy J. Correlation between aromatase expression in the eutopic endometrium of symptomatic patients and the presence of endometriosis. Int J Women’s Health. 2012;4:61–5.
Kitawaki J, Kado N, Ishihara H, Koshiba H, Kitaoka Y, Honjo H. Endometriosis: the pathophysiology as an estrogen-dependent disease. J Steroid Biochem Mol Biol. 2002;83:149–55.
Ezaki K, Motoyama H, Sasaki H. Immunohistologic localization of estrone sulfatase in uterine endometrium and adenomyosis. Obstet Gynecol. 2001;98:815–9.
Urabe M, Yamamoto T, Kitawaki J, Honjo H, Okada H. Estrogen biosynthesis in human uterine adenomyosis. Acta Endocrinol (Copenh). 1989;121(2):259–64.
Zeng YY, Guan YG, Li KY. Role of estrogen, estrogen receptors, and aromatase in the pathogenesis of uterine adenomyosis. Nan Fang Yi Ke Da Xue Xue Bao. 2017;37(3):383–7.
Zhao L, Zhou S, Zou L, Zhao X. The expression and functionality of stromal caveolin 1 in human adenomyosis. Hum Reprod. 2013;28(5):1324–38.
Donnez J, Stratopoulou CA, Dolmans MM. Uterine adenomyosis: from disease pathogenesis to a new medical approach using GnRH antagonists. Int J Environ Res Public Health. 2021;18(19):9941.
Mehasseb MK, Panchal R, Taylor AH, Brown L, Bell SC, Habiba M. Estrogen and progesterone receptor isoform distribution through the menstrual cycle in uteri with and without adenomyosis. Fertil Steril. 2011;95(7):2228-2235.el.
Li Y, Zou S, Xia X, Zhang S. Human adenomyosis endometrium stromal cells secreting more nerve growth factor: impact and effect. Reprod Sci. 2015;22(9):1073–82.
Guo SW, Mao X, Ma Q, Liu X. Dysmenorrhea and its severity are associated with increased uterine contractility and overexpression of oxytocin receptor (OTR) in women with symptomatic adenomyosis. Fertil Steril. 2013;99(1):231–40.
Nie J, Liu X, Guo SW. Immunoreactivity of oxytocin receptor and transient receptor potential vanilloid type 1 and its correlation with dysmenorrhea in adenomyosis. Am J Obstet Gynecol. 2010;202(4):346.e1-8.
Duckitt K. Menorrhagia. BMJ. Clin Evid. 2015;2015:0805.
Orazov MR, Nosenko EN, Radzinsky VE, Khamoshina MB, Lebedeva MG, Sounov MA. Proangiogenic features in chronic pelvic pain caused by adenomyosis. Gynecol Endocrinol. 2016;32:7–10.
Guo S, Zhang D, Lu X, Zhang Q, Gu R, Sun B, et al. Hypoxia and its possible relationship with endometrial receptivity in adenomyosis: a preliminary study. Reprod Biol Endocrinol. 2021;19(1):7.
Quiñonez-Flores CM, González-Chávez SA, Pacheco-Tena C. Hypoxia and its implications in rheumatoid arthritis. J Biomed Sci. 2016;23(1):62.
Huang TS, Chen YJ, Chou TY, Chen CY, Li HY, Huang BS, et al. Oestrogen-induced angiogenesis promotes adenomyosis by activating the Slug-VEGF axis in endometrial epithelial cells. J Cell Mol Med. 2014;18(7):1358–71.
Karel C, Michal J, Radovan P, Pavel V, Jana Ž, Jan V, et al. Adenomyosis - its possible effect on endometrial function and receptivity. Ceska Gynekol. 2021;86(3):205–9.
Patterson AL, Pirochta J, Tufano SY, Teixeira JM. Gain-of-function beta-catenin in the uterine mesenchyme leads to impaired implantation and decidualization. J Endocrinol. 2017;233(1):119–30.
Peng Y, Jin Z, Liu H, Xu C. Impaired decidualization of human endometrial stromal cells from women with adenomyosis. Biol Reprod. 2021;104(5):1034–44.
Ashary N, Laheri S, Modi D. Homeobox genes in endometrium: from development to decidualization. Int J Dev Biol. 2020;64(1-2–3):227–37.
Wang J, Huang C, Jiang R, Du Y, Zhou J, Jiang Y, et al. Decreased endometrial il-10 impairs endometrial receptivity by downregulating HOXA10 expression in women with adenomyosis. Biomed Res Int. 2018;2018:2549789.
Nusse R. Wnt signaling. Cold Spring Harb Perspect Biol. 2012;4:a011163.
Odia Y, Cavalcante L, Safran H, Powell SF, Munster PN, Ma WW, et al. Malignant glioma subset from actuate 1801: phase I/II study of 9-ING-41, GSK-3β inhibitor, monotherapy or combined with chemotherapy for refractory malignancies. Neurooncol Adv. 2022;74(1):vdac012.
Li X, Liu Y, Zhao Y, Tian W, Zhai L, Pang H, et al. Rhein derivative 4F inhibits the malignant phenotype of breast cancer by downregulating Rac1 protein. Front Pharmacol. 2020;11:754.
Zhou G, Peng F, Zhong Y, Chen Y, Tang M, Li D. Rhein suppresses matrix metalloproteinase production by regulating the Rac1/ROS/MAPK/AP-1 pathway in human ovarian carcinoma cells. Int J Oncol. 2017;50(3):933–41.
Feng T, Wei S, Wang Y, Fu X, Shi L, Qu L, Fan X. Rhein ameliorates adenomyosis by inhibiting NF-kappaB and beta-catenin signaling pathway. Biomed Pharmacother. 2017;94:231–7.
Massagué J. TGFbeta in Cancer. Cell. 2008;134(2):215–30.
Melzer C, Hass R, von der Ohe J, Lehnert H, Ungefroren H. The role of TGF-beta and its crosstalk with RAC1/RAC1b signaling in breast and pancreas carcinoma. Cell Commun Signal. 2017;15(1):19.
Patel S, Tang J, Overstreet JM, Anorga S, Lian F, Arnouk A, et al. Rac-GTPase promotes fibrotic TGF-beta1 signaling and chronic kidney disease via EGFR, p53, and Hippo/YAP/TAZ pathways. FASEB J. 2019;33(9):9797–810.
Rasmi RR, Sakthivel KM, Guruvayoorappan C. NF-kappaB inhibitors in treatment and prevention of lung cancer. Biomed Pharmacother. 2020;130:110569.
Burgos RA, Alarcón P, Quiroga J, Manosalva C, Hancke J. Andrographolide, an anti-inflammatory multitarget drug: all roads lead to cellular metabolism. Molecules. 2020;26(1):5.
Liu L, Luo N, Guo J, Xie Y, Chen L, Cheng Z. Berberine inhibits growth and inflammatory invasive phenotypes of ectopic stromal cells: imply the possible treatment of adenomyosis. J Pharmacol Sci. 2018;137(1):5–11.
Ortiz LM, Lombardi P, Tillhon M, Scovassi AI. Berberine, an epiphany against cancer. Molecules. 2014;19(8):12349–67.
Yang JH, Wu MY, Chen MJ, Chen SU, Yang YS, Ho HN. Increased matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-1 secretion but unaffected invasiveness of endometrial stromal cells in adenomyosis. Fertil Steril. 2009;91(5 Suppl):2193–8.
Vannuccini S, Luisi S, Tosti C, Sorbi F, Petraglia F. Role of medical therapy in the management of uterine adenomyosis. Fertil Steril. 2018;109(3):398–405.
Zhu B, Chen Y, Shen X, Liu X, Guo SW. Anti-platelet therapy holds promises in treating adenomyosis: experimental evidence. Reprod Biol Endocrinol. 2016;14(1):66.
Zhang X, Li M, Zuo K, Li D, Ye M, Ding L, et al. Upregulated miR-155 in papillary thyroid carcinoma promotes tumor growth by targeting APC and activating Wnt/beta-catenin signaling. J Clin Endocrinol Metab. 2013;98(8):E1305-1313.
Vasquez YM, Mazur EC, Li X, Kommagani R, Jiang L, Chen R, et al. FOXO1 is required for binding of PR on IRF4, novel transcriptional regulator of endometrial stromal decidualization. Mol Endocrinol. 2015;29(3):421–33.
Acknowledgements
I thank Mrs. Toyomi Kobayashi for creating the figure.
Author information
Authors and Affiliations
Contributions
HK: conception and design, acquisition of data, analysis and interpretation of data, and writing the manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Patient Consent for Publication
Not applicable.
Competing interests
The author declares no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kobayashi, H. Molecular Targets for Nonhormonal Treatment Based on a Multistep Process of Adenomyosis Development. Reprod. Sci. 30, 743–760 (2023). https://doi.org/10.1007/s43032-022-01036-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-022-01036-4