Abstract
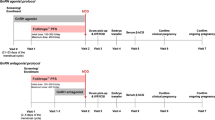
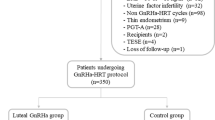
Changes in coagulation and fibrinolysis have been reported in women undergoing controlled ovarian hyperstimulation (COH) supporting a potential hypercoagulable condition related to this treatment. This study aimed at evaluating the changes in fibrin clot properties and thrombin generation induced by two different COH protocols: long with gonadotropin-releasing hormone agonist (GnRH-a) and GnRH antagonist (GnRH-ant). Primary outcomes included determination of plasma fibrin clot properties, including clot permeability (Ks) and efficiency of fibrinolysis using clot lysis time (CLT), along with thrombin generation (prothrombin fragments 1 + 2) and endogenous thrombin potential (ETP) and fibrinolysis inhibitor levels. One hundred twenty-nine infertile women were included in the final analysis. The GnRH-ant protocol resulted in increased ETP (+ 9.8%) and reduced Ks (− 2.4%). Conversely, COH with the GnRH-a protocol reduced thrombin generation by decreasing both ETP (− 6.6%) and F1 + 2 (− 30.8%) together with favorably altered fibrin clot properties represented by increased Ks (+ 21.7%) and reduced CLT (− 13.8%) as well as decreased PAI-1 levels (by 2.5 times). The GnRH-ant compared to the GnRH-a protocol increased PAI-1 levels (+ 77.3%), thrombin generation (9.3% higher ETP), and Ks (+ 13.7%). In the GnRH-a group, post-COH Ks was 14.3% higher (Ks ≥ 7.92 × 10−9 cm2) in women with positive vs. negative pregnancy outcomes. Our results show that the GnRH-ant protocol enhanced thrombin generation and slightly decreased fibrin clot density. COH with the GnRH-a reduced thrombin generation and improved fibrin clot features. This trial was registered (NCT04166825). Clinical Trial Registration Number: NCT04166825.




Similar content being viewed by others
Data Availability
All data are stored in Gynecological Endocrinology Department and available at request.
Code Availability
iCentrum software.
Abbreviations
- COH:
-
Controlled ovarian hyperstimulation
- PCOS:
-
Polycystic ovary syndrome
References
Wang R, Lin S, Wang Y, Qian W, Zhou L. Comparisons of GnRH antagonist protocol versus GnRH agonist long protocol in patients with normal ovarian reserve: a systematic review and meta-analysis. PLoS ONE. 2017;12(4):e0175985. https://doi.org/10.1371/journal.pone.0175985.
Lambalk CB, Banga FR, Huirne JA, Toftager M, Pinborg A, Homburg R, et al. GnRH antagonist versus long agonist protocols in IVF: a systematic review and meta-analysis accounting for patient type. Hum Reprod Update. 2017;23(5):560–79. https://doi.org/10.1093/humupd/dmx017.
Bates SM. Anticoagulation and in vitro fertilization and ovarian stimulation. Hematol Am Soc Hematol Educ Program. 2014;2014(1):379–86. https://doi.org/10.1182/asheducation-2014.1.379.
Gerotziafas GT, Van Dreden P, Mathieu d’Argent E, Lefkou E, Grusse M, Comtet M, et al. Impact of blood hypercoagulability on in vitro fertilization outcomes: a prospective longitudinal observational study. Thromb J. 2017;15:9. https://doi.org/10.1186/s12959-017-0131-7.
Sticchi E, Romagnuolo I, Cellai AP, Lami D, Fedi S, Prisco D, et al. Fibrinolysis alterations in infertile women during controlled ovarian stimulation: influence of BMI and genetic components. Thromb Res. 2012;130(6):919–24. https://doi.org/10.1016/j.thromres.2012.07.005.
Group ECW. Venous thromboembolism in women: a specific reproductive health risk. Hum Reprod Update. 2013;19(5):471–82. https://doi.org/10.1093/humupd/dmt028.
Balandina AN, Koltsova EM, Teterina TA, Yakovenko AG, Simonenko EU, Poletaev AV, et al. An enhanced clot growth rate before in vitro fertilization decreases the probability of pregnancy. PLoS ONE. 2019;14(5):e0216724. https://doi.org/10.1371/journal.pone.0216724.
Ferraretti AP, Gianaroli L. The Bologna criteria for the definition of poor ovarian responders: is there a need for revision? Hum Reprod. 2014;29(9):1842–5. https://doi.org/10.1093/humrep/deu139.
Pankiw-Bembenek O, Zalewski J, Goralczyk T, Undas A. A history of early stent thrombosis is associated with prolonged clot lysis time. Thromb Haemost. 2012;107(3):513–20. https://doi.org/10.1160/TH11-09-0662.
Undas A, Zawilska K, Ciesla-Dul M, Lehmann-Kopydłowska A, Skubiszak A, Ciepłuch K, et al. Altered fibrin clot structure/function in patients with idiopathic venous thromboembolism and in their relatives. Blood. 2009;114(19):4272–8. https://doi.org/10.1182/blood-2009-05-222380.
Pieters M, Philippou H, Undas A, de Lange Z, Rijken DC, Mutch NJ, et al. An international study on the feasibility of a standardized combined plasma clot turbidity and lysis assay: communication from the SSC of the ISTH. J Thromb Haemost. 2018;16(5):1007–12. https://doi.org/10.1111/jth.14002.
Knol HM, Kemperman RF, Kluin-Nelemans HC, Mulder AB, Meijer K. Haemostatic variables during normal menstrual cycle. A systematic review Thromb Haemost. 2012;107(1):22–9. https://doi.org/10.1160/TH11-07-0481.
Koh SC, Prasad RN, Fong YF. Hemostatic status and fibrinolytic response potential at different phases of the menstrual cycle. Clin Appl Thromb Hemost. 2005;11(3):295–301. https://doi.org/10.1177/107602960501100308.
Govorov I, Bremme K, Lindahl TL, Holmström M, Komlichenko E, Chaireti R, et al. Thrombin generation during a regular menstrual cycle in women with von Willebrand disease. Sci Rep. 2018;8(1):17467. https://doi.org/10.1038/s41598-018-35897-0.
Huang Y, Zhao Y, Yan L, Chuai YH, Liu LL, Chen Y, et al. Changes in coagulation and fibrinolytic indices in women with polycystic ovarian syndrome undergoing controlled ovarian hyperstimulation. Int J Endocrinol. 2014;2014:731498 https://doi.org/10.1155/2014/731498
Godtfredsen ACM, Sidelmann JJ, Gram JB, Andersen M, Glintborg D. Fibrin lysability is associated with central obesity and inflammation in women with polycystic ovary syndrome. Acta Obstet Gynecol Scand. 2020;99(8):1078–84. https://doi.org/10.1111/aogs.13825.
Yamaguti EM, Brito MB, Ferriani RA, Garcia AA, Rosa-e-Silva JC, Vieira CS. Comparison of the hemostatic effects of a levonorgestrel-releasing intrauterine system and leuprolide acetate in women with endometriosis: a randomized clinical trial. Thromb Res. 2014;134(6):1193–7. https://doi.org/10.1016/j.thromres.2014.09.014.
Piróg M, Kacalska-Janssen O, Jach R, Ząbczyk M, Natorska J. Fibrin clot properties among women with endometriosis and the impact of ovarian stimulation. Reprod Biomed Online. 2021;43(1):81–90. https://doi.org/10.1016/j.rbmo.2021.03.008.
Beck-Fruchter R, Gavish I, Baram S, Geslevich Y, Weiss A. Increased coagulation index as measured by thromboelastography during ovarian stimulation for in vitro fertilization: Influence of the final oocyte maturation triggering agent. Eur J Obstet Gynecol Reprod Biol. 2018;223:26–9. https://doi.org/10.1016/j.ejogrb.2018.02.005.
Westerlund E, Henriksson P, Wallén H, Hovatta O, Wallberg KR, Antovic A. Detection of a procoagulable state during controlled ovarian hyperstimulation for in vitro fertilization with global assays of haemostasis. Thromb Res. 2012;130(4):649–53. https://doi.org/10.1016/j.thromres.2011.11.024.
Romagnuolo I, Sticchi E, Fedi S, Cellai AP, Lami D, Alessandrello Liotta A, et al. Is tissue factor pathway inhibitor a marker of procoagulable status in healthy infertile women undergoing ovarian stimulation for assisted reproduction? Blood Coagul Fibrinolysis. 2014;25(3):254–8. https://doi.org/10.1097/MBC.0000000000000044.
Cohen Y, Tulandi T, Almog B, Zohav E, Deutsch V, Many A, et al. Prolonged activation of the coagulation system during in vitro fertilization cycles. Eur J Obstet Gynecol Reprod Biol. 2017;216:111–5. https://doi.org/10.1016/j.ejogrb.2017.07.021.
Harnett MJ, Bhavani-Shankar K, Datta S, Tsen LC. In vitro fertilization-induced alterations in coagulation and fibrinolysis as measured by thromboelastography. Anesth Analg. 2002;95(4):1063–6, table of contents. https://doi.org/10.1097/00000539-200210000-00050
Orbach-Zinger S, Eidelman LA, Lutsker A, Oron G, Fisch B, Ben-Haroush A. The effect of in vitro fertilization on coagulation parameters as measured by thromboelastogram. Eur J Obstet Gynecol Reprod Biol. 2016;201:118–20. https://doi.org/10.1016/j.ejogrb.2016.04.010.
Magnani B, Tsen L, Datta S, Bader A. In vitro fertilization. Do short-term changes in estrogen levels produce increased fibrinolysis? Am J Clin Pathol. 1999;112(4):485–91. https://doi.org/10.1093/ajcp/112.4.485.
Aune B, Høie KE, Oian P, Holst N, Osterud B. Does ovarian stimulation for in-vitro fertilization induce a hypercoagulable state? Hum Reprod. 1991;6(7):925–7. https://doi.org/10.1093/oxfordjournals.humrep.a137461.
Siudut J, Grela M, Wypasek E, Plens K, Undas A. Reduced plasma fibrin clot permeability and susceptibility to lysis are associated with increased risk of postthrombotic syndrome. J Thromb Haemost. 2016;14(4):784–93. https://doi.org/10.1111/jth.13264.
Szczepaniak P, Zabczyk M, Undas A. Increased plasma clot permeability and susceptibility to lysis are associated with heavy menstrual bleeding of unknown cause: a case-control study. PLoS ONE. 2015;10(4):e0125069. https://doi.org/10.1371/journal.pone.0125069.
Lockwood CJ, Krikun G, Rahman M, Caze R, Buchwalder L, Schatz F. The role of decidualization in regulating endometrial hemostasis during the menstrual cycle, gestation, and in pathological states. Semin Thromb Hemost. 2007;33(1):111–7. https://doi.org/10.1055/s-2006-958469.
Schatz F, Guzeloglu-Kayisli O, Arlier S, Kayisli UA, Lockwood CJ. The role of decidual cells in uterine hemostasis, menstruation, inflammation, adverse pregnancy outcomes and abnormal uterine bleeding. Hum Reprod Update. 2016;22(4):497–515. https://doi.org/10.1093/humupd/dmw004.
Sarto A, Rocha M, Martínez M, Sergio PR. Hypofibrinolysis and other hemostatic defects in women with antecedents of early reproductive failure. Medicina (B Aires). 2000;60(4):441–7.
Martínez-Zamora MA, Creus M, Tassies D, Reverter JC, Civico S, Carmona F, et al. Reduced plasma fibrinolytic potential in patients with recurrent implantation failure after IVF and embryo transfer. Hum Reprod. 2011;26(3):510–6. https://doi.org/10.1093/humrep/deq369.
Acknowledgements
We would like to thank Prof. Anetta Undas for the contribution to this work.
Funding
This work was supported by a grant of the Polish National Science Center (UMO-2016/21/N/NZ4/03269 to MP).
Author information
Authors and Affiliations
Contributions
M.P.: preparation of samples, experimental design, data collection and analysis, drafting the manuscript; O.K-J.: experimental design and interpretation of data; R.J.: interpretation of data and analysis of the manuscript; J.W. data collection, interpretation of data; B.C. data collection, interpretation of data; M.Z.: laboratory investigations, statistical analysis, interpretation of data; J.N.: laboratory investigations, interpretation of data and drafting the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The Ethics Committee at Jagiellonian University Medical College in Krakow approved the study (date of approval: 18 December 2014 and extension of approval: 30 September 2018; reference number KBET/292/B/2014).
Consent to Participate
All participants provided informed consent in accordance with the Declaration of Helsinki.
Consent for Publication
All patients included in this study had signed the approved informed consent to allow publication of anonymous data.
Competing Interests
The authors declare no competing interests.
Additional information
Headings: A comparison between two controlled ovarian hyperstimulation (COH) protocols: long with gonadotropin-releasing hormone agonist (GnRH-a) and GnRH antagonist (GnRH-ant), focusing on the impact on thrombin generation, fibrinolysis, and fibrin clot properties. .
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Piróg, M., Kacalska-Janssen, O., Jach, R. et al. GnRH Antagonist Protocol Enhances Coagulation During Controlled Ovarian Stimulation for IVF. Reprod. Sci. 29, 3521–3531 (2022). https://doi.org/10.1007/s43032-022-01026-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-022-01026-6