Abstract
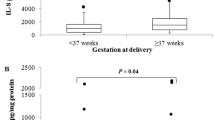
Inflammatory processes associated with human parturition are still not completely understood, not only because the gap between inflammation and the onset of labor has been difficult to study but also because of the limited knowledge about the role of cervicovaginal fluid (CVF) cytokines during the sequence of labor. We aimed to determine whether CVF cytokines could predict the onset of normal and preterm labor. Chemokines and proinflammatory and anti-inflammatory cytokines in CVF were measured in a pseudo-longitudinal manner in healthy women between 12 and 41 weeks gestation with intact fetal membranes before and during the first stage of labor. Women were grouped into five stages, from the absence of uterine activity and cervical changes to regular uterine contractions with cervix dilation > 3 cm (active phase of labor). Of 144 women with spontaneous labor, 96 gave birth at term, 48 gave birth preterm, and both groups displayed similar cytokine concentrations. We found positive correlations between proinflammatory cytokines and the initial sequence of labor, using individual cytokines and score-based data by principal component analysis (IFN-γ, TNF-α, IL-1β, IL-6) as dependent variables. The risk of labor onset increased as the concentrations of IL-6 increased (hazard ratio = 202.09, 95% confidence interval = 24.57–1662.49, P < 0.001). The IL-6 concentration predicted the onset of labor within 12 days of sampling (area under the time-dependent ROC curve = 0.785, 95% confidence interval = 0.693–0.877). Here, we showed that regardless of gestational age, the onset of labor could be predicted by the IL-6 concentration in the CVF, since the initial sequence of spontaneous labor displayed an inflammatory response expressed by the increase in proinflammatory cytokines.







Similar content being viewed by others
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
References
Li T, Yi T, Zhao J, Zhao X, He X. Combined proinflammatory biomarkers have better predictive value for term labor than single markers. Med Sci Monit. 2019;25:4513–20.
Unal ER, Cierny JT, Roedner C, Newman R, Goetzl L. Maternal inflammation in spontaneous term labor. Am J Obstet Gynecol. 2011;204:223.e1-223.e5.
Gomez-Lopez N, Vega-Sanchez R, Castillo-Castrejon M, Romero R, Cubeiro-Arreola K, Vadillo-Ortega F. Evidence for a role for the adaptive immune response in human term parturition. Am J Reprod Immunol. 2013;69:212–30.
Gomez-Lopez N, StLouis D, Lehr MA, Sanchez-Rodriguez EN, Arenas-Hernandez M. Immune cells in term and preterm labor. Cell Mol Immunol. 2014;11:571–81.
Menon R, Bonney EA, Condon J, Mesiano S, Taylor RN. Novel concepts on pregnancy clocks and alarms: redundancy and synergy in human parturition. Hum Reprod Update. 2016;22:535–60.
Young A, Thomson AJ, Ledingham M, Jordan F, Greer IA, Norman JE. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol Reprod. 2002;66:445–9.
Osman I, Young A, Ledingham MA, et al. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod. 2003;9:41–5.
Gomez-Lopez N, Motomura K, Miller D, Garcia-Flores V, Galaz J, Romero R. Inflammasomes: their role in normal and complicated pregnancies. J Immunol. 2019;203(11):2757–69.
Motomura K, Romero R, García-Flores V, et al. The alarmin interleukin-1α causes preterm birth through the NLRP3 inflammasome. Mol Hum Reprod. 2020;26(9):712–26.
Faro J, Romero R, Schwenkel G, et al. Intra-amniotic inflammation induces preterm birth by activating the NLRP3 inflammasome. Biol Reprod. 2019;100(5):1290–305.
Kim SA, Park KH, Lee SM, Kim YM, Hong S. Inflammatory proteins in the amniotic fluid, plasma, and cervicovaginal fluid for the prediction of intra-amniotic infection/inflammation and imminent preterm birth in preterm labor. Am J Perinatol. 2020.https://doi.org/10.1055/s-0040-1718575
Stranik J, Kacerovsky M, Andrys C, et al. Intra-amniotic infection and sterile intra-amniotic inflammation are associated with elevated concentrations of cervical fluid interleukin-6 in women with spontaneous preterm labor with intact membranes. J Matern Fetal Neonatal Med. 2021;7:1–9.
Park JW, Park KH, Lee SY. Noninvasive prediction of intra-amniotic infection and/or inflammation in women with preterm labor: various cytokines in cervicovaginal fluid. Reprod Sci. 2013;20(3):262–8.
Holst RM, Hagberg H, Wennerholm UB, et al. Prediction of spontaneous preterm delivery in women with preterm labor: analysis of multiple proteins in amniotic and cervical fluids. Obstet Gynecol. 2009;114(2 Pt 1):268–77.
Holst RM, Mattsby-Baltzer I, Wennerholm UB, et al. Interleukin-6 and interleukin-8 in cervical fluid in a population of Swedish women in preterm labor: relationship to microbial invasion of the amniotic fluid, intra-amniotic inflammation, and preterm delivery. Acta Obstet Gynecol Scand. 2005;84(6):551–7.
Abalos E, Chamillard M, Díaz V, Pasquale J, Souza JP. Progression of the first stage of spontaneous labour. Best Pract Res Clin Obstet Gynaecol. 2020;67:19–32.
Harrell FEJ, Slaughter JC. Biostatistics for biomedical research. Power. 2015;1–158.
Romero R, Durum S, Dinarello CA, Oyarzun E, Hobbins JC, Mitchell MD. Interleukin-1 stimulates prostaglandin biosynthesis by human amnion. Prostaglandins. 1989;37:13–22.
Hertelendy F, Romero R, Molnar M, Todd H, Baldassare JJ. Cytokine-initiated signal transduction in human myometrial cells. Am J Reprod Immunol. 1993;30:49–57.
Belt AR, Baldassare JJ, Molna´r M, Romero R, Hertelendy F. The nuclear transcription factor NF-kappaB mediates interleukin-1 beta-induced expression of cyclooxygenase-2 in human myometrial cells. Am J Obstet Gynecol. 1999;181:359–66.
Sivarajasingam SP, Imami N, Johnson MR. Myometrial cytokines and their role in the onset of labour. J Endocrinol. 2016;231(3):R101–19.
Vadillo-Ortega F, Estrada-Gutiérrez G. Role of matrix metalloproteinases in preterm labour. BJOG. 2005;112(Suppl 1):19–22.
Combs CA, Gravett M, Garite TJ, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;210:125.e1-125.e15.
Oh KJ, Kim SM, Hong JS, et al. Twenty-four percent of patients with clinical chorioamnionitis in preterm gestations have no evidence of either culture-proven intraamniotic infection or intraamniotic inflammation. Am J Obstet Gynecol. 2017;216:604.e1-604.e11.
Romero R, Miranda J, Chaiworapongsa T, et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol. 2014;72:458–74.
Bollapragada S, Youssef R, Jordan F, Greer I, Norman J, Nelson S. Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. Am J Obstet Gynecol. 2009;200(1):104.e1-11.
Seong HS, Lee SE, Kang JH, Romero R, Yoon BH. The frequency of microbial invasion of the amniotic cavity and histologic chorioamnionitis in women at term with intact membranes in the presence or absence of labor. Am J Obstet Gynecol. 2008;199(4):375.e1-5.
Lucaroni F, Morciano L, Rizzo G, et al. Biomarkers for predicting spontaneous preterm birth: an umbrella systematic review. J Matern Fetal Neonatal Med. 2018;31(6):726–34.
Monastero RN, Pentyala S. Cytokines as biomarkers and their respective clinical cutoff levels. Int J Inflam. 2017;2017:4309485.
Conde-Agudelo A, Papageorghiou AT, Kennedy SH, Villar J. Novel biomarkers for the prediction of the spontaneous preterm birth phenotype: a systematic review and meta-analysis. BJOG. 2011;118(9):1042–54.
Lamont RF, Richardson LS, Boniface JJ, et al. Commentary on a combined approach to the problem of developing biomarkers for the prediction of spontaneous preterm labor that leads to preterm birth. Placenta. 2020;98:13–23.
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG practice bulletin, number 234. Obstet Gynecol 2021;138(2):e65.
Santhanam U, Avila C, Romero R, et al. Cytokines in normal and abnormal parturition: elevated amniotic fluid interleukin-6 levels in women with premature rupture of membranes associated with intrauterine infection. Cytokine. 1991;3:155–63.
Menon R, Torloni MR, Voltolini C, et al. Biomarkers of spontaneous preterm birth: an overview of the literature in the last four decades. Reprod Sci. 2011;18:1046–70.
Committee Opinion No. 712: Intrapartum management of intraamniotic infection. Obstet Gynecol. 2017;130(2):e95–101.
Higgins RD, Saade G, Polin RA, et al. Chorioamnionitis workshop participants. Evaluation and management of women and newborns with a maternal diagnosis of chorioamnionitis: summary of a workshop. Obstet Gynecol. 2016;127(3):426–36.
Fettweis JM, Serrano MG, Brooks JP, et al. The vaginal microbiome and preterm birth. Nat Med. 2019;25(6):1012–21.
Genser B, Cooper PJ, Yazdanbakhsh M, Barreto ML, Rodrigues LC. A guide to modern statistical analysis of immunological data. BMC Immunol. 2007;26(8):27.
Ballenberger N, Lluis A, von Mutius E, Illi S, Schaub B. Novel statistical approaches for non-normal censored immunological data: analysis of cytokine and gene expression data. PLoS ONE. 2012;7(10): e46423.
Heng YJ, Liong S, Permezel M, Rice GE, Di Quinzio MK, Georgiou HM. The interplay of the interleukin 1 system in pregnancy and labor. Reprod Sci. 2014;21(1):122–30.
Heng YJ, Di Quinzio MK, Permezel M, Rice GE, Georgiou HM. Interleukin-1 receptor antagonist in human cervicovaginal fluid in term pregnancy and labor. Am J Obstet Gynecol. 2008;199(6):656.e1-7.
Funding
Funding for this project was provided by Fundación Gonzalo Río Arronte (S.633), SECTEI/253/2019 and Fundación Mexicana para la Salud (Proyecto Interinstitucional). DESC received fellowship (1046662) from the Mexican Council of Science and Technology (CONACyT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
This study was approved by the Health Ministry institutional IRB (Register 2010/010/3117, 9 August 2017) and by the INMEGEN IRB (12/2018/I) in accordance with the Declaration of Helsinki and the Guideline for Good Clinical Practices. All participants signed informed consent.
Consent for Publication
All authors agree to participate in this publication. This study does not include images or other personal or clinical details of participants that compromise anonymity. With their consent to participate, all patients have also signed a written consent to publish their data anonymously.
Competing Interests
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sandoval-Colin, D.E., Godines-Enriquez, M.S., Espejel-Núñez, A. et al. Cervicovaginal Cytokines to Predict the Onset of Normal and Preterm Labor: a Pseudo-longitudinal Study. Reprod. Sci. 30, 221–232 (2023). https://doi.org/10.1007/s43032-022-01007-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-022-01007-9