Abstract
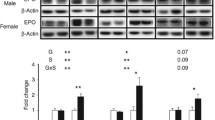
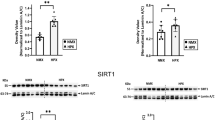
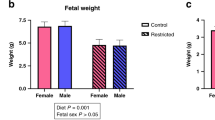
Chronic hypoxia can cause fetal growth restriction (FGR) through placental dysfunction. Insulin-like growth factors (IGFs), such as IGF-2, play a major role in preservation of placental growth and function. We investigated the effects of chronic hypoxia and sex on protein expression of the IGF-2 pathway in placentas selected from asymmetric-FGR fetuses. Time-mated pregnant guinea pigs were assigned to normoxia (NMX, 21% O2) or hypoxia (HPX, 10.5% O2) during the last 14 days of pregnancy. Placentas were selected from male and female symmetrically grown NMX fetuses (fetal wt between 25th ile–75th ile) and HPX fetuses of asymmetric-FGR (fetal wt < 25th ile and brain:liver wt > 50th ile). Effects of HPX and sex on placenta protein expression of the IGF-2 signaling proteins (IGF-2, PI3K, AKT-P, total AKT, PCNA, a cell proliferation marker) were evaluated by immunoblotting. Effects of HPX and sex on morphometric parameters were analyzed using two-way ANOVA (p < 0.05). HPX reduced (p < 0.005) fetal wt by ~ 35% compared to NMX in both sexes. Expression of IGF-2 was lower (p = 0.029) in NMX female placentas compared to males. Despite lower NMX levels, HPX increased (p < 0.05) expression of IGF-2, AKT-P, relative AKT-P, and PCNA in female placentas only and had no effect on protein expression in male placentas. The female guinea pig placenta exhibits a greater sensitivity than males to HPX in upregulating expression of the IGF-2 axis. In addition, the sex difference in baseline IGF-2 expression suggests a greater capacity for females to increase IGF-2 in response to HPX as a placental adaptation in FGR.





Similar content being viewed by others
References
Gordijn SJ, Beune IM, Thilaganatha B, Papageorghiou A, Baschat AA, Baker PN, Silver RM, Wynia K, Ganzevoort W. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol. 2016;48(3):333–9. https://doi.org/10.1002/uog.15884.
Burton, GJ, Fowden AL. Review: the placenta and developmental programming: balancing fetal nutrient demands with maternal resource allocation. Placenta, 2012; S23–7 https://doi.org/10.1016/j.placenta.2011.11.013
Vaughan OR, Sferruzzi-Perri AN, Coan PM, Fowden AL. Environmental regulation of placental phenotype: implications for fetal growth. Reprod Fertil Dev. 2011;24(1):80–96. https://doi.org/10.1071/RD11909.
Parraguez VH, Atlagich M, Diaz R, Cepeda R, Gonzalez C, Reyes MD, Bruzzone ME, Behn C, Raggi LA. Ovine placenta at high altitudes: comparison of animals with different times of adaptation to hypoxic environment. Anim Reprod Sci. 2006;95(1–2):151–7. https://doi.org/10.1016/j.anireprosci.2005.11.003.
Jansson N, Pettersson J, Haffiz A, Erricson A, Palmberg I, Tranberg M, Ganapathy V, Powell TL, Jansson T. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J Physiol. 2006;576(Pt 3):935–46. https://doi.org/10.1113/jphysiol.2006.116509.
Nardozza LM, Caetano AC, Zamarian AC, Mazzola JB, Silva CP, Marcal VM, Lobo TF, Peixoto AB, Junior EA. Fetal growth restriction: current knowledge. Arch Gynecol Obstet. 2017;295(5):1061–77. https://doi.org/10.1007/s00404-017-4341-9.
Soares MJ, Iqbal K, Kozai K. Hypoxia and placental development. Birth Defects Res. 2017;109(17):1309–29. https://doi.org/10.1002/bdr2.1135.
Semenza GL. Oxygen homeostasis. Wiley Interdiscip Rev Syst Biol Med. 2010;2(3):336–61. https://doi.org/10.1056/NEJMra1011165.
Hutter D, Kingdom J, Jaeggi E. Causes and mechanisms of intrauterine hypoxia and its impact on the fetal cardiovascular system: a review. Int J Pediatr. 2010;2010:4013–23. https://doi.org/10.1155/2010/401323.
Börzsönyi B, Demendi C, Nagy Z, Toth K, Csanad M, Pajor A, Rig J, Joo JG. Gene expression patterns of insulin-like growth factor 1, insulin-like growth factor 2 and insulin-like growth factor binding protein 3 in human placenta from pregnancies with intrauterine growth restriction. J Perinat Med. 2011;39(6):701–7. https://doi.org/10.1515/jpm.2011.090.
Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75(1):73–82. https://doi.org/10.1016/S0092-8674(05)80085-6
Powell-Braxton L, Hollingshead P, Warburton C, Dowd M, Pitts-Meek S, Dalton D, Gillett N, Stewart TA. IGF-I is required for normal embryonic growth in mice. Genes Dev. 1993;7(12B):2609–17. https://doi.org/10.1101/gad.7.12b.2609.
DeChiara TM, Efstratiadis A, Robertson EJ. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature. 1990;345(6270):78–80. https://doi.org/10.1038/345078a0.
Sheikh S, Satoskar P, Bhartiya D. Expression of insulin-like growth factor-I and placental growth hormone mRNA in placentae: a comparison between normal and intrauterine growth retardation pregnancies. Mol Hum Reprod. 2001;7(3):287–92. https://doi.org/10.1093/molehr/7.3.287.
Abu-Amero SN, Ali Z, Bennett P, Vaughan JI, Moore GE. Expression of the insulin-like growth factors and their receptors in term placentas: a comparison between normal and IUGR births. Mol Reprod Dev. 1998;49(3):229–35. https://doi.org/10.1002/(SICI)1098-2795(199803)49:3%3c229::AID-MRD2%3e3.0.CO;2-Q.
Calvo MT, Romo A, Gutierrez JJ, Relano E, Barrio E, Longas AF. Study of genetic expression of intrauterine growth factors IGF-I and EGFR in placental tissue from pregnancies with intrauterine growth retardation. J Pediatr Endocrinol Metab. 2004;17(Suppl 3):445–50. https://doi.org/10.1186/s13148-016-0178-5
Koukoura O, Sifakis S, Soufla G, Zaravinos A, Apostolidou S, Jones A, Widschwendter M, Spandidos DA. Loss of imprinting and aberrant methylation of IGF2 in placentas from pregnancies complicated with fetal growth restriction. Int J Mol Med. 2011;28(4):481–7. https://doi.org/10.3892/ijmm.2011.754.
Vincent AM, Feldman EL. Control of cell survival by IGF signaling pathways. Growth Horm IGF Res. 2002;12(4):193–7. https://doi.org/10.1016/S1096-6374(02)00017-5.
Daoud G, Amyot M, Rassart E, Masse A, Simoneau L, Lafond J. ERK1/2 and p38 regulate trophoblasts differentiation in human term placenta. J Physiol. 2005;566(Pt 2):409–23. https://doi.org/10.1113/jphysiol.2005.089326.
Chen J, Yue C, Xu J, Zhan Y, Zhao H, Li Y, Ye Y. Downregulation of receptor tyrosine kinase-like orphan receptor 1 in preeclampsia placenta inhibits human trophoblast cell proliferation, migration, and invasion by PI3K/AKT/mTOR pathway accommodation. Placenta. 2019;82:17–24. https://doi.org/10.1016/j.placenta.2019.05.002.
Diaz LE, Chuan YC, Lewitt M, Fernandez-Perez L, Carrasco-Rodriguez S, Sanchez-Gomez M, Flores-Morales A. IGF-II regulates metastatic properties of choriocarcinoma cells through the activation of the insulin receptor. Mol Hum Reprod. 2007;13(8):567–76. https://doi.org/10.1093/molehr/gam039.
Ain R, Canham LN, Soares MJ. Dexamethasone-induced intrauterine growth restriction impacts the placental prolactin family, insulin-like growth factor-II and the Akt signaling pathway. J Endocrinol. 2005;185(2):253–63. https://doi.org/10.1677/joe.1.06039.
Higgins JS, Vaughan OR, de Fernandez Liger E, Fowden AL, Sferruzzi-Perri AN. Placental phenotype and resource allocation to fetal growth are modified by the timing and degree of hypoxia during mouse pregnancy. J Physiol. 2016;594(5):1341–56. https://doi.org/10.1113/JP271057.
Trollmann R, Klingmuller K, Schild RL, Rascher W, Dotsch J. Differential gene expression of somatotrophic and growth factors in response to in vivo hypoxia in human placenta. Am J Obstet Gynecol. 2007;197(6):601.e1-6. https://doi.org/10.1016/j.ajog.2007.04.008.
Sferruzzi-Perri AN. Regulating needs: exploring the role of insulin-like growth factor-2 signalling in materno-fetal resource allocation. Placenta. 2018;64(Suppl 1):S16–22. https://doi.org/10.1016/j.placenta.2018.01.005.
Matthews PJ, Jackson J. Pregnancy diagnosis in the guinea pig. Lab Anim Sci. 1977;27(2):248–50.
Thompson LP, Dong Y, Evans L. Chronic hypoxia increases inducible NOS-derived nitric oxide in fetal guinea pig hearts. Pediatr Res. 2009;65(2):188–92. https://doi.org/10.1203/PDR.0b013e31818d6ad0.
Oh C, Dong Y, Liu H, Thompson LP. Intrauterine hypoxia upregulates proinflammatory cytokines and matrix metalloproteinases in fetal guinea pig hearts. Am J Obstet Gynecol. 2008;199(1):78.e1-6. https://doi.org/10.1016/j.ajog.2007.12.004.
Turan S, Aberdeen GW, Thompson LP. Chronic hypoxia alters maternal uterine and fetal hemodynamics in the full-term pregnant guinea pig. Am J Physiol Regul Integr Comp Physiol. 2017;313(4):R330–9. https://doi.org/10.1152/ajpregu.00056.2017.
Coan PM, Angiolini E, Sandovici I, Burton GJ, Costancia M. Fowden AL Adaptations in placental nutrient transfer capacity to meet fetal growth demands depend on placental size in mice. J Physiol. 2008;586(18):4567–76. https://doi.org/10.1113/jphysiol.2008.156133.
Elias AA, Ghaly A, Matushewski B, Regnault TRH. Richardson, Maternal nutrient restriction in guinea pigs as an animal model for inducing fetal growth restriction. Reprod Sci. 2016;23(2):219–27. https://doi.org/10.1177/1933719115602773.
Ghaly A, Maki Y, Nygard K, Hammond R, Hardy DB, Richardson BS. Maternal nutrient restriction in guinea pigs leads to fetal growth restriction with increased brain apoptosis. Pediatr Res. 2019;85(1):105–12. https://doi.org/10.1038/s41390-018-0230-6.
Song H, Telugu BP, Thompson LP. Sexual dimorphism of mitochondrial function in the hypoxic guinea pig placenta. Biol Reprod. 2019;100(1):208–16. https://doi.org/10.1093/biolre/ioy167.
Carter AM, Kingston MJ, Han KK, Mazzuca DM, Nygard K, Han VK. Altered expression of IGFs and IGF-binding proteins during intrauterine growth restriction in guinea pigs. J Endocrinol. 2005;184(1):179–89. https://doi.org/10.1677/joe.1.05781.
Coan PM, Vaughan OR, Sekita Y, Finn SL, Burton GJ, Costancia M, Fowden AL. Adaptations in placental phenotype support fetal growth during undernutrition of pregnant mice. J Physiol. 2010;588(Pt 3):527–38. https://doi.org/10.1113/jphysiol.2009.181214.
Constância M, Angiolini E, Sandovici I, Smith P, Smith R, Kelsey G, Dean W, Ferguson-Smith A, Sibley C, Reik W, Fowden AL. Adaptation of nutrient supply to fetal demand in the mouse involves interaction between the Igf2 gene and placental transporter systems. Proc Natl Acad Sci U S A. 2005;102(52):19219–24. https://doi.org/10.1073/pnas.0504468103.
Gratton RJ, Asano H, Han VK. The regional expression of insulin-like growth factor II (IGF-II) and insulin-like growth factor binding protein-1 (IGFBP-1) in the placentae of women with pre-eclampsia. Placenta. 2002;23(4):303–10. https://doi.org/10.1053/plac.2001.0780.
Sferruzzi-Perri AN, Vaughan OR, Coan PM, Suciu MC, Darbyshire R, Constancia M, Burton GJ, Fowden AL. Placental-specific Igf2 deficiency alters developmental adaptations to undernutrition in mice. Endocrinology. 2011;152(8):3202–12. https://doi.org/10.1210/en.2011-0240.
Han VK, Bassett N, Walton J, Challis JR. The expression of insulin-like growth factor (IGF) and IGF-binding protein (IGFBP) genes in the human placenta and membranes: evidence for IGF-IGFBP interactions at the feto-maternal interface. J Clin Endocrinol Metab. 1996;81(7):2680–93. https://doi.org/10.1210/jcem.81.7.8675597.
Kind KL, Roberts CT, Sohlstrom AI, Katsman A, Clifton PM, Robinson JS, Owens JA. Chronic maternal feed restriction impairs growth but increases adiposity of the fetal guinea pig. Am J Physiol Regul Integr Comp Physiol. 2005;288(1):R119–26. https://doi.org/10.1152/ajpregu.00360.2004.
Roberts CT, Sohlstrom AI, Kind KL, Earl RA, Khong TY, Robinson JS, Owens PC, Owens JA. Maternal food restriction reduces the exchange surface area and increases the barrier thickness of the placenta in the guinea-pig. Placenta. 2001;22(2–3):177–85. https://doi.org/10.1053/plac.2000.0602.
Sharma D, Shastri S, Farahbakhsh N, Sharma P. Intrauterine growth restriction - part 1. J Matern Fetal Neonatal Med. 2016;29(24):3977–87. https://doi.org/10.3109/14767058.2016.1152249.
Sharma D, Shastri S, Farahbakhsh N, Sharma P. Intrauterine growth restriction - part 2. J Matern Fetal Neonatal Med. 2016;29(24):4037–48. https://doi.org/10.3109/14767058.2016.1154525.
Feldser D, Agani F, Iyer NV, Pak B, Ferriera G, Semenza GL. Reciprocal positive regulation of hypoxia-inducible factor 1alpha and insulin-like growth factor 2. Cancer Res. 1999;59(16):3915–8.
Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev. 1998;8(5):588–94. https://doi.org/10.1016/S0959-437X(98)80016-6.
Pringle KG, Kind KL, Thompson JG, Roberts CT. Complex interactions between hypoxia inducible factors, insulin-like growth factor-II and oxygen in early murine trophoblasts. Placenta. 2007;28(11–12):1147–57. https://doi.org/10.1016/j.placenta.2007.05.009.
Kent LN, Ohboshi S, Soares MJ. Akt1 and insulin-like growth factor 2 (Igf2) regulate placentation and fetal/postnatal development. Int J Dev Biol. 2012;56(4):255–61. https://doi.org/10.1387/ijdb.113407lk.
Yang ZZ, Tschopp O, Hemmings-Mieszczak M, Feng J, Brodbeck D, Perentes E, Hemmings BA. Protein kinase B alpha/Akt1 regulates placental development and fetal growth. J Biol Chem. 2003;278(34):32124–31. https://doi.org/10.1074/jbc.M302847200.
Foukas LC, Claret M, Pearce W, Okkenhaug K, Meek S, Peskett E, Sancho S, Smith AJ, Withers DJ, Vanhaeebroeck B. Critical role for the p110alpha phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature. 2006;441(7091):366–70. https://doi.org/10.1038/nature04694.
Sferruzzi-Perri AN, Lopez-Tello J, Fowden AL, Constancia M. Maternal and fetal genomes interplay through phosphoinositol 3-kinase(PI3K)-p110α signaling to modify placental resource allocation. Proc Natl Acad Sci U S A. 2016;113(40):11255–60. https://doi.org/10.1073/pnas.1602012113.
Thompson LP, Pence L, Pinkas G, Song H, Telugu B. Placental hypoxia during early pregnancy causes maternal hypertension and placental insufficiency in the hypoxic guinea pig model. Biol Reprod. 2016;95(6):128. https://doi.org/10.1095/biolreprod.116.142273.
Zhang MS, Hu AH, Qiu H, Xiong HH, Chen Y. The correlation between IGF-II and Bcl-2 expression in colorectal adenocarcinoma. Med Oncol. 2012;29(2):928–32. https://doi.org/10.1007/s12032-011-9881-4.
Kuang RG, Wu HX, Hao GX, Wang JW, Zhou CJ. Expression and significance of IGF-2, PCNA, MMP-7, and α-actin in gastric carcinoma with Lauren classification. Turk J Gastroenterol. 2013;24(2):99–108. https://doi.org/10.4318/tjg.2013.0571.
Radford EJ, Isganaitis E, Jimenez-Chillaron J, Schroeder J, Molla M, Andrews S, Didier N, Charalambous M, McEwen K, Marrazzi G, Sassoon D, Patti ME, Ferguson-Smith AC. An unbiased assessment of the role of imprinted genes in an intergenerational model of developmental programming. PLoS Genet. 2012;8(4):e1002605. https://doi.org/10.1371/journal.pgen.1002605.
Mina TH, Raikkonen K, Riley SC, Norman JE, Reynolds RM. Maternal distress associates with placental genes regulating fetal glucocorticoid exposure and IGF2: role of obesity and sex. Psychoneuroendocrinology. 2015;59:112–22. https://doi.org/10.1016/j.psyneuen.2015.05.004.
Cuffe JS, Walton SL, Singh RR, Spiers JG, Bielefeldt-Ohmann H, Wilkinson L, Little MH, Moritz KM. Mid- to late term hypoxia in the mouse alters placental morphology, glucocorticoid regulatory pathways and nutrient transporters in a sex-specific manner. J Physiol. 2014;592(14):3127–41. https://doi.org/10.1113/jphysiol.2014.272856.
Meakin AS, Saif Z, Jones AR, Aviles PFV, Clifton VL. Review: placental adaptations to the presence of maternal asthma during pregnancy. Placenta. 2017;54:17–23. https://doi.org/10.1016/j.placenta.2017.01.123.
Sferruzzi-Perri AN, Sandovici I, Constancia M, Fowden AL. Placental phenotype and the insulin-like growth factors: resource allocation to fetal growth. J Phyiosl. 2017;595(15):5057–93. https://doi.org/10.1113/JP273330.
Stevenson DK, Verter J, Fanaroff AA, Oh W, Ehrenkranz RA, Shankaran S, Donovan EF, Wright LL, Lemons JA, Tyson JE, Korones SB, Bauer CR, Stoll BJ, Papile LA. Sex differences in outcomes of very low birthweight infants: the newborn male disadvantage. Arch Dis Child Fetal Neonatal Ed. 2000;83(3):F182–5. https://doi.org/10.1136/fn.83.3.f182.
Murphy VE, Gibson PG, Giles WB, Zakar T, Smith R, Bisits AM, Kessell CG, Clifton VL. Maternal asthma is associated with reduced female fetal growth. Am J Respir Crit Care Med. 2003;168(11):1317–23. https://doi.org/10.1164/rccm.200303-374OC.
Stark MJ, Clifton VL, Wright IM. Neonates born to mothers with preeclampsia exhibit sex-specific alterations in microvascular function. Pediatr Res. 2009;65(3):292–5. https://doi.org/10.1203/pdr.0b013e318193edf1.
Thompson LP, Turan S, Aberdeen GW. Sex differences and the effects of intrauterine hypoxia on growth and in vivo heart function of fetal guinea pigs. Am J Physiol Regul Integr Comp Physiol. 2020;319(3):R243–54. https://doi.org/10.1152/ajpregu.00249.2019.
Stark MJ, Clifton VL, Wright IM. Microvascular flow, clinical illness severity and cardiovascular function in the preterm infant. Arch Dis Child Fetal Neonatal Ed. 2008;93(4):F271–4. https://doi.org/10.1136/adc.2007.123539.
Funding
The project described is supported in part by a National Institute of Health (NIH HL126859, LPT) grant. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institute of Health.
All animal procedures were approved by the University of Maryland Animal Care and Use Committee in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care-accredited procedures (Animal Welfare Assurance No. A3200-01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
43032_2022_979_MOESM1_ESM.pptx
Supplementary file1 Figure 1. Western immunoblot of IGF-2 (MW=13kDa) expression in male and female placentas. Normoxic (NMX) (top) andhypoxic (HPX, 10.5% O2, 14 d) (bottom) placentas were loadedonto separate gels to compare differences due to sex. Each lane represents asingle placenta sample (N=7 for each group).Each target band was normalized to ß-actin (MW= 42 kDa) as a loadingcontrol. (PPTX 408 KB)
43032_2022_979_MOESM2_ESM.pptx
Supplementary file2 Figure 2. Western immunoblot of IGF-2 protein (MW=13kDa)expression in normoxic and hypoxic (10.5%O2, 14d) placentas. Male (top) and female (bottom)placentas were loaded onto separate gelsto compare the effects of treatment.Each lane represents a single placenta sample (N=7 for each). Each target band was normalized to ß-actin (MW=42 kDa) as a loading control. (PPTX 456 KB)
43032_2022_979_MOESM3_ESM.pptx
Supplementary file3 Figure 3. Western immunoblot ofphosphoinositide 3-kinase (PI3K, MW= 84 kDa) expression in normoxic and hypoxic(10.5%O2, 14d) placentas. Male(top) and female (bottom) placentas were loaded onto separategels to compare the effects of treatment.Each lane represents a single placenta sample (N=7 for each). Each target band was normalized to ß-actin (MW= 42 kDa) as a loading control. (PPTX 415 KB)
43032_2022_979_MOESM4_ESM.pptx
Supplementary file4 Figure 4. Western immunoblot of phosphorylated(AKT-P) and total protein kinase B (AKT-Total) expression in normoxic andhypoxic (10.5%O2, 14d) placentas.Male (top two images) and female (bottomtwo images) placentas were loaded onto separate gels to compare th effects oftreatment. For both male and femalesamples, membranes were first probed for AKT-P (MW=57kDa), stripped, and thenreprobed for AKT-Total (MW=57kDa). Each target band was normalized to ß-actin (MW= 42 kDa) as a loading control to obtain values for AKT-P and AKT-Total. Subsequently, AKT-P values were normalized toAKT-Total. Each lane represents a singleplacenta sample (N=7 for each group). (PPTX 803 KB)
43032_2022_979_MOESM5_ESM.pptx
Supplementary file5 Figure 5. Western immunoblot of proliferating cellnuclear antigen (PCNA, MW = 29kDa) expression in normoxic and hypoxic (10.5%O2,14d) placentas. Male (top) andfemale (bottom) placentas were loaded onto separate gels to compare theeffects of treatment. Each lanerepresents a single placenta sample (N=7 for each). Each target band was normalized to ß-actin (MW= 42 kDa) as a loading control. (PPTX 364 KB)
43032_2022_979_MOESM6_ESM.pptx
Supplementary file6 Figure 6: Western immunoblot offull membrane targeting IGF-2 and Beta actin of normoxic and hypoxic (10.5%O2,14d) male placentas. Top membrane identified IGF-2 targeted with a polyclonalantibody at a MW of 13kDa. Bottom imageidentifies the same membrane was stripped and re-probed for Beta actinidentified at a MW of 42kDa. (PPTX 1408 KB)
Rights and permissions
About this article
Cite this article
Elsamadicy, E.A., Thompson, L.P. Sex-Selective Increase of IGF-2 Expression in the Hypoxic Guinea Pig Placenta of Growth-Restricted Fetuses. Reprod. Sci. 29, 3015–3025 (2022). https://doi.org/10.1007/s43032-022-00979-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-022-00979-y