Abstract
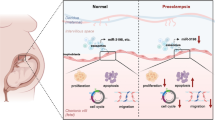
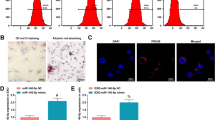
Preeclampsia (PE) is a pregnancy complication with high maternal and fetal morbidity and mortality rates. During pregnancy, the concentration of exosomes in the maternal blood circulation would increase, establishing that plasma exosomes play a role in the development of pregnancy. Our previous study implied the important role of exosomal miR-199a-5p in preeclampsia with severe features (sPE). This study aims to reveal the role of exosomal miR-199a-5p in contribution to the development of sPE. The results showed that the expression of miR-199a-5p was significantly higher in plasma exosomes and placenta tissue from patients with sPE than that in normal pregnant women. Additionally, hydrogen peroxide (H2O2) could upregulate the expression of miR-199a-5p in BeWo cells and cell-derived exosomes. In terms of the regulatory effect, exosomal miR-199a-5p was observed to inhibit the expression of SIRT1 in human umbilical venous endothelial cells (HUVECs). Moreover, the treatment of both miR-199a-5p-overexpressed exosomes and SIRT1 inhibitor EX527 could decrease the nitric oxide production, elevate the intracellular reactive oxygen species level, and enhance the expressions of ICAM-1 and VCAM-1 of HUVECs. Thus, our findings suggest that the upregulated plasma exosomal miR-199a-5p in sPE might result from the trophoblast of the impaired placenta under oxidative stress. Furthermore, exosomal miR-199a-5p could impair the endothelial cell function via targeting SIRT1, contributing to the development of preeclampsia.






Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
Change history
12 December 2023
A Correction to this paper has been published: https://doi.org/10.1007/s43032-023-01416-4
References
Phipps E, Prasanna D, Brima W, Jim B. Preeclampsia: updates in pathogenesis, definitions, and guidelines. Clin J Am Soc Nephrol. 2016;11(6):1102–13. https://doi.org/10.2215/CJN.12081115.
Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet Gynecol. 2020;135(6):e237-e60https://doi.org/10.1097/AOG.0000000000003891
Croke L. Gestational hypertension and preeclampsia: a practice bulletin from ACOG. Am Fam Physician. 2019;100(10):649–50.
Tomimatsu T, Mimura K, Matsuzaki S, Endo M, Kumasawa K, Kimura T. Preeclampsia: maternal systemic vascular disorder caused by generalized endothelial dysfunction due to placental antiangiogenic factors. Int J Mol Sci. 2019;20(17). https://doi.org/10.3390/ijms20174246.
Buhimschi IA, Nayeri UA, Zhao G, Shook LL, Pensalfini A, Funai EF et al. Protein misfolding, congophilia, oligomerization, and defective amyloid processing in preeclampsia. Sci Transl Med. 2014;6(245):245ra92. https://doi.org/10.1126/scitranslmed.3008808.
Verma S, Anderson TJ. Fundamentals of endothelial function for the clinical cardiologist. Circulation. 2002;105(5):546–9.
Konukoglu D, Uzun H. Endothelial dysfunction and hypertension. Adv Exp Med Biol. 2017;956:511–40. https://doi.org/10.1007/5584_2016_90.
Boeldt DS, Bird IM. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J Endocrinol. 2017;232(1):R27–44. https://doi.org/10.1530/JOE-16-0340.
Wortzel I, Dror S, Kenific CM, Lyden D. Exosome-mediated metastasis: communication from a distance. Dev Cell. 2019;49(3):347–60.
Czernek L, Duchler M. Exosomes as messengers between mother and fetus in pregnancy. Int J Mol Sci. 2020;21(12). https://doi.org/10.3390/ijms21124264.
Sarker S, Scholz-Romero K, Perez A, Illanes SE, Mitchell MD, Rice GE, et al. Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J Transl Med. 2014;12:204. https://doi.org/10.1186/1479-5876-12-204.
Pillay P, Maharaj N, Moodley J, Mackraj I. Placental exosomes and pre-eclampsia: maternal circulating levels in normal pregnancies and early and late onset pre-eclamptic pregnancies. Placenta. 2016;46:18–25. https://doi.org/10.1016/j.placenta.2016.08.078.
Fabian MR, Sonenberg N. The mechanics of miRNA-mediated gene silencing: a look under the hood of miRISC. Nat Struct Mol Biol. 2012;19(6):586–93. https://doi.org/10.1038/nsmb.2296.
Sun Z, Shi K, Yang S, Liu J, Zhou Q, Wang G, et al. Effect of exosomal miRNA on cancer biology and clinical applications. Mol Cancer. 2018;17(1):147. https://doi.org/10.1186/s12943-018-0897-7.
Greening DW, Gopal SK, Xu R, Simpson RJ, Chen W. Exosomes and their roles in immune regulation and cancer. Semin Cell Dev Biol. 2015;40:72–81. https://doi.org/10.1016/j.semcdb.2015.02.009.
Mycko MP, Baranzini SE. microRNA and exosome profiling in multiple sclerosis. Mult Scler. 2020;26(5):599–604. https://doi.org/10.1177/1352458519879303.
Zhu J, Liu B, Wang Z, Wang D, Ni H, Zhang L, et al. Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation. Theranostics. 2019;9(23):6901–19. https://doi.org/10.7150/thno.37357.
Chen Z, Zhang W, Wu M, Huang H, Zou L, Luo Q. Pathogenic mechanisms of preeclampsia with severe features implied by the plasma exosomal miRNA profile. Bioengineered. 2021. https://doi.org/10.1080/21655979.2021.1993717.
Ministrini S, Puspitasari YM, Beer G, Liberale L, Montecucco F, Camici GG. Sirtuin 1 in endothelial dysfunction and cardiovascular aging. Front Physiol. 2021;12: 733696. https://doi.org/10.3389/fphys.2021.733696.
Zhao Y, Zheng YF, Luo QQ, Yan T, Liu XX, Han L, et al. Edaravone inhibits hypoxia-induced trophoblast-soluble Fms-like tyrosine kinase 1 expression: a possible therapeutic approach to preeclampsia. Placenta. 2014;35(7):476–82. https://doi.org/10.1016/j.placenta.2014.04.002.
Dong Y, Chi X, Hai H, Sun L, Zhang M, Xie WF, et al. Antibodies in the breast milk of a maternal woman with COVID-19. Emerg Microbes Infect. 2020;9(1):1467–9. https://doi.org/10.1080/22221751.2020.1780952.
Yoshida K, Tsuda M, Matsumoto R, Semba S, Wang L, Sugino H, et al. Exosomes containing ErbB2/CRK induce vascular growth in premetastatic niches and promote metastasis of bladder cancer. Cancer Sci. 2019;110(7):2119–32. https://doi.org/10.1111/cas.14080.
Lu R-H, Xiao Z-Q, Zhou J-D, Yin C-Q, Chen Z-Z, Tang F-J et al. MiR-199a-5p represses the stemness of cutaneous squamous cell carcinoma stem cells by targeting Sirt1 and CD44ICD cleavage signaling. Cell cycle (Georgetown, Tex). 2020;19(1). https://doi.org/10.1080/15384101.2019.1689482.
Hua Q, Jin M, Mi B, Xu F, Li T, Zhao L, et al. LINC01123, a c-Myc-activated long non-coding RNA, promotes proliferation and aerobic glycolysis of non-small cell lung cancer through miR-199a-5p/c-Myc axis. J Hematol Oncol. 2019;12(1):91. https://doi.org/10.1186/s13045-019-0773-y.
Li Y, Wang D, Li X, Shao Y, He Y, Yu H, et al. MiR-199a-5p suppresses non-small cell lung cancer via targeting MAP3K11. J Cancer. 2019;10(11):2472–9. https://doi.org/10.7150/jca.29426.
Yang GR, Dye TD, Li D. Effects of pre-gestational diabetes mellitus and gestational diabetes mellitus on macrosomia and birth defects in Upstate New York. Diabetes Res Clin Pract. 2019;155: 107811. https://doi.org/10.1016/j.diabres.2019.107811.
Redman CWG, Staff AC. Preeclampsia, biomarkers, syncytiotrophoblast stress, and placental capacity. American journal of obstetrics and gynecology. 2015;213(4 Suppl):S9.e1, S9-S.e1, 11. https://doi.org/10.1016/j.ajog.2015.08.003.
Brooks D, Barr LC, Wiscombe S, McAuley DF, Simpson AJ, Rostron AJ. Human lipopolysaccharide models provide mechanistic and therapeutic insights into systemic and pulmonary inflammation. Eur Respir J. 2020;56(1). https://doi.org/10.1183/13993003.01298-2019.
Coyle CH, Martinez LJ, Coleman MC, Spitz DR, Weintraub NL, Kader KN. Mechanisms of H2O2-induced oxidative stress in endothelial cells. Free Radic Biol Med. 2006;40(12):2206–13. https://doi.org/10.1016/j.freeradbiomed.2006.02.017.
Yang XZ, Chang Y, Wei W. Endothelial dysfunction and inflammation: immunity in rheumatoid arthritis. Med Inflam 2016;2016:1–9.
Cyr AR, Huckaby LV, Shiva SS, Zuckerbraun BS. Nitric oxide and endothelial dysfunction. Crit Care Clin. 2020;36(2):307–21. https://doi.org/10.1016/j.ccc.2019.12.009.
Incalza MA, D'Oria R, Natalicchio A, Perrini S, Laviola L, Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol. 2018;100. https://doi.org/10.1016/j.vph.2017.05.005.
Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, et al. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103(9):1282–8.
Radi R. Oxygen radicals, nitric oxide, and peroxynitrite: redox pathways in molecular medicine. Proc Natl Acad Sci USA. 2018;115(23):5839–48. https://doi.org/10.1073/pnas.1804932115.
Cheang WS, Wong WT, Wang L, Cheng CK, Lau CW, Ma RCW, et al. Resveratrol ameliorates endothelial dysfunction in diabetic and obese mice through sirtuin 1 and peroxisome proliferator-activated receptor δ. Pharmacol Res. 2019;139:384–94. https://doi.org/10.1016/j.phrs.2018.11.041.
Zhang H-N, Dai Y, Zhang C-H, Omondi AM, Ghosh A, Khanra I, et al. Sirtuins family as a target in endothelial cell dysfunction: implications for vascular ageing. Biogerontology. 2020;21(5):495–516. https://doi.org/10.1007/s10522-020-09873-z.
Kitada M, Ogura Y, Koya D. The protective role of Sirt1 in vascular tissue: its relationship to vascular aging and atherosclerosis. Aging (Albany NY). 2016;8(10):2290–307. https://doi.org/10.18632/aging.101068.
Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23(12):2369–80.
Mattagajasingh I, Kim C-S, Naqvi A, Yamamori T, Hoffman TA, Jung S-B, et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 2007;104(37):14855–60.
Evans CE, Iruela-Arispe ML, Zhao YY. Mechanisms of endothelial regeneration and vascular repair and their application to regenerative medicine. Am J Pathol. 2021;191(1):52–65. https://doi.org/10.1016/j.ajpath.2020.10.001.
Funding
This work was supported by Natural Science Foundation of Sichuan Province (No.2022NSFSC1373), the Luzhou Key R&D Technology Project for Social Development (No. 2021-syf-27), the Doctoral Research Initiation Fund of Affiliated Hospital of Southwest Medical University, and the National Natural Science Foundation of China (No. 81703242).
Author information
Authors and Affiliations
Contributions
All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission. Li Zou and Qingqing Luo contributed to the study design. Zhirui Chen performed experiments and analyzed data. Zhirui Chen, Qingqing Luo, Mengying Wu, Haixia Huang, and Hui Tao contributed to the drafting and revision of the manuscript. Qingqing Luo contributed to funding acquisition.
Corresponding authors
Ethics declarations
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology (HUST) (No. 2018S419).
Consent to Participate
Not applicable.
Consent for Publication
All the authors agreed to submit the manuscript for the possible publication of the journal.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Z., Wu, M., Huang, H. et al. Plasma Exosomal miR-199a-5p Derived from Preeclampsia with Severe Features Impairs Endothelial Cell Function via Targeting SIRT1. Reprod. Sci. 29, 3413–3424 (2022). https://doi.org/10.1007/s43032-022-00977-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-022-00977-0