Abstract
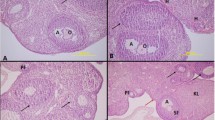
There is still controversy over whether structural and morphological changes can be observed in tissues depending on the carbon dioxide (CO2) nature or the applied intra-abdominal pressures (IAP). This study aimed to investigate the effects of different pressure or CO2 nature used for pneumoperitoneum in gynecological laparoscopic surgery on inflammation, DNA damage, oxidative stress, and histopathological changes in ovarian and peritoneal tissue. For this purpose, forty female rats were randomly divided into 6 groups and different pneumoperitoneum models were created in these groups. Rats in group other than control and sham groups received standard (CD) or heated-humidified CO2 (HH) insufflations at low (4 mmHg) or high pressure (8 mmHg). The ovary and peritoneum sections were evaluated microscopically for apoptotic index (API) and API scoring was calculated. Tissue and plasma interleukin-6 (IL-6), tumor necrotizing factor-alpha (TNF-α), anti-Mullerian hormone (AMH) and 8-hydroxydeoxyguanosine (8-OHdG) levels were analyzed with enzyme-linked immunosorbent assay (ELISA). The most severe changes occurred in the 8CD group microscopically, while the least severe changes occurred in the 4HH group. All histopathological parameters except for ovarian apoptotic index and peritoneal PCNA at low pressure were higher in the CD group. TNF-α and 8-OHdG levels were higher in the CD group at both low and high pressures. Standard CO2 caused more prominent histopathological changes at high pressures and systemic inflammation in both pressure groups. The least change between the experimental study groups in terms of histopathological and biochemical was observed in the low-pressure heated-humidified group.



Similar content being viewed by others
Abbreviations
- PCNA:
-
Proliferating cell nuclear antigen
- INOS:
-
Inducible nitric oxide synthase
- IL-6:
-
Interleukin 6
- TNF-α:
-
Tumor necrosis factor α
- AMH:
-
Anti-Mullerian hormone
- 8-OHdG:
-
8-Hydroxy-2′-deoxyguanosine
References
Sajid MS, Mallick AS, Rimpel J, et al. Effect of heated and humidified carbon dioxide on patients after laparoscopic procedures: a meta-analysis. Surg Laparosc Endosc Percutan Tech. 2008;18:539–46. https://doi.org/10.1097/SLE.0b013e3181886ff4.
Matsuzaki S, Vernis L, Bonnin M, et al. Effects of low intraperitoneal pressure and a warmed, humidified carbon dioxide gas in laparoscopic surgery: a randomized clinical trial. Sci Rep. 2017;7(1):11287. https://doi.org/10.1038/s41598-017-10769-1.
Binda MM. Humidification during laparoscopic surgery: overview of the clinical benefits of using humidified gas during laparoscopic surgery. Arch Gynecol Obstet. 2015;292(5):955–71. https://doi.org/10.1007/s00404-015-3717-y.
Peng Y, Zheng M, Ye Q, et al. Heated and humidified CO2 prevents hypothermia, peritoneal injury, and intra-abdominal adhesions during prolonged laparoscopic insufflations. J Surg Res. 2009;151(1):40–7. https://doi.org/10.1016/j.jss.2008.03.039.
Bashirov E, Cetiner S, Emre M, et al. A randomized controlled study evaluating the effects of the temperature of insufflated CO2 on core body temperature and blood gases (an experimental study). Surg Endosc. 2007;21(10):1820–5. https://doi.org/10.1007/s00464-007-9295-8.
Neuhaus SJ, Watson DI. Pneumoperitoneum and peritoneal surface changes: a review. Surg Endosc. 2004;18(9):1316–22. https://doi.org/10.1007/s00464-003-8238-2.
Sammour T, Mittal A, Delahunt B, et al. Warming and humidification have no effect on oxidative stress during pneumoperitoneum in rats. Minim Invasive Ther Allied Technol. 2011;20(6):329–37. https://doi.org/10.3109/13645706.2011.556647.
Hazebroek EJ, Schreve MA, Visser P, et al. Impact of temperature and humidity of carbon dioxide pneumoperitoneum on body temperature and peritoneal morphology. J Laparoendosc Adv Surg Tech A. 2002;12(5):355–64. https://doi.org/10.1089/109264202320884108.
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal Research. PLoS Biol. 2010;8(6):e1000412. https://doi.org/10.1371/journal.pbio.1000412.
Akdemir A, Erbaş O, Ergenoğlu M, et al. Montelukast prevents ischaemia/reperfusion-induced ovarian damage in rats. Eur J Obstet Gynecol Reprod Biol. 2014;173:71–6. https://doi.org/10.1016/j.ejogrb.2013.11.021.
Avital S, Itah R, Szomstein S, et al. Correlation of CO2 pneumoperitoneal pressures between rodents and humans. Surg Endosc. 2009;23(1):50–4. https://doi.org/10.1007/s00464-008-9862-7.
Davey AK, Hayward J, Marshall JK, Woods AE. The effects of insufflation conditions on rat mesothelium. Int J Inflam. 2013;2013:816283. https://doi.org/10.1155/2013/816283.
Volz J, Köster S, Spacek Z, Paweletz N. Characteristic alterations of the peritoneum after carbon dioxide pneumoperitoneum. Surg Endosc. 1999;13(6):611–4. https://doi.org/10.1007/s004649901052.
Gurel C, Kuscu GC, Buhur A, et al. Fluvastatin attenuates doxorubicin-induced testicular toxicity in rats by reducing oxidative stress and regulating the blood-testis barrier via mTOR signaling pathway. Hum Exp Toxicol. 2019;38(12):1329–43. https://doi.org/10.1177/0960327119862006.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. https://doi.org/10.1006/abio.1976.9999.
Brunner E, Konietschke F, Pauly M, Puri ML. Rank-based procedures in factorial designs: hypotheses about non-parametric treatment effects. J R Statist Soc B. 2017;79(5):1463–85. https://doi.org/10.1111/rssb.12222.
Kuhry E, Jeekel J, Bonjer HJ. Effect of laparoscopy on the immune system. Semin Laparosc Surg. 2004;11(1):37–44. https://doi.org/10.1177/107155170401100107.
Birch DW, Dang JT, Switzer NJ, Manouchehri N, Shi X, Hadi G, Karmali S. Heated insufflation with or without humidification for laparoscopic abdominal surgery. Cochrane Database Syst Rev. 2016;10(10):CD007821. https://doi.org/10.1002/14651858.CD007821.pub3.
Matsuzaki S, Jardon K, Maleysson E, et al. Impact of intraperitoneal pressure of a CO2 pneumoperitoneum on the surgical peritoneal environment. Hum Reprod. 2012;27(6):1613–23. https://doi.org/10.1093/humrep/des081.
Matsuzaki S, Jardon K, Maleysson E, et al. Carbon dioxide pneumoperitoneum, intraperitoneal pressure, and peritoneal tissue hypoxia: a mouse study with controlled respiratory support. Surg Endosc. 2010;24(11):2871–80. https://doi.org/10.1007/s00464-010-1069-z.
Kıray S, Onalan G, Karabay G, Zeyneloglu H, Kuscu E. Antioxidant prophylaxis for cellular injury in ovarian surface epithelium resulting from CO2 pneumoperitoneum in a laparoscopic rat model. Arch Gynecol Obstet. 2011;284(3):765–72. https://doi.org/10.1007/s00404-011-1933-7.
Sakin O, Anğın AD, Akalın EE, et al. Comparison of the protective effects of sildenafil, vardenafil and tadalafil treatments in ischemia-reperfusion injury in rat ovary. Ginekol Pol. 2019;90(9):513–9. https://doi.org/10.5603/GP.2019.0089.
Boehm EM, Gildenberg MS, Washington MT. The many roles of PCNA in eukaryotic DNA replication. Enzymes. 2016;39:231–54. https://doi.org/10.1016/bs.enz.2016.03.003.
Ugurel V, CagatayCicek A, Cemek M, et al. Antioxidant and antiapoptotic effects of erdosteine in a rat model of ovarian ischemia-reperfusion injury. Iran J Basic Med Sci. 2017;20(1):53–8. https://doi.org/10.22038/ijbms.2017.8093.
Pergel A, Kanter M, Yucel AF, et al. Anti-inflammatory and antioxidant effects of infliximab in a rat model of intestinal ischemia/reperfusion injury. Toxicol Ind Health. 2012;28(10):923–32. https://doi.org/10.1177/0748233711427056.
Refaie MMM, El-Hussieny M. Protective effect of pioglitazone on ovarian ischemia reperfusion injury of female rats via modulation of peroxisome proliferator activated receptor gamma and heme-oxygenase 1. Int Immunopharmacol. 2018;62:7–14. https://doi.org/10.1016/j.intimp.2018.06.037.
Ali FF, Ahmed AF, Elroby Ali DM. Underlying mechanisms behind the protective effect of angiotensin (1–7) in experimental rat model of ovarian ischemia reperfusion injury. Life Sci. 2019;235:116840. https://doi.org/10.1016/j.lfs.2019.116840.
Saed GM, Abu-Soud HM, Diamond MP. Role of nitric oxide in apoptosis of human peritoneal and adhesion fibroblasts after hypoxia. Fertil Steril. 2004;82(SUPPL. 3):1198–205. https://doi.org/10.1016/j.fertnstert.2004.04.034.
Papparella A, Noviello C, Romano M, et al. Local and systemic impact of pneumoperitoneum on prepuberal rats. Pediatr Surg Int. 2007;23(5):453–7. https://doi.org/10.1007/s00383-006-1860-z.
Papparella A, Nino F, Coppola S, et al. Peritoneal morphological changes due to pneumoperitoneum: the effect of intra-abdominal pressure. Eur J Pediatr Surg. 2013;24(4):322–7. https://doi.org/10.1055/s-0033-1349057.
Puttick MI, Scott-Coombes DM, Dye J, et al. Comparison of immunologic and physiologic effects of CO2 pneumoperitoneum at room and body temperatures. Surg Endosc. 1999;13(6):572–5. https://doi.org/10.1007/s004649901043.
Sammour T, Kahokehr A, Hayes J, Hulme-Moir M, Hill AG. Warming and humidification of insufflation carbon dioxide in laparoscopic colonic surgery: a double-blinded randomized controlled trial. Ann Surg. 2010;251(6):1024–33. https://doi.org/10.1097/SLA.0b013e3181d77a25.
Margulis V, Matsumoto ED, Tunc L, et al. Effect of warmed, humidified insufflation gas and anti-inflammatory agents on cytokine response to laparoscopic nephrectomy: porcine model. J Urol. 2005;174(4 Pt 1):1452–6. https://doi.org/10.1097/01.ju.0000173011.81396.12.
McMillen MA, Huribal M, Sumpio B. Common pathway of endothelial-leukocyte interaction in shock, ischemia, and reperfusion. Am J Surg. 1993;166(5):557–62. https://doi.org/10.1016/s0002-9610(05)81153-5.
Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. https://doi.org/10.1016/j.biocel.2006.07.001.
Cooke MS, Evans MD, Dove R, et al. DNA repair is responsible for the presence of oxidatively damaged DNA lesions in urine. Mutat Res. 2005;574(1–2):58–66. https://doi.org/10.1016/j.mrfmmm.2005.01.022.
Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2’ - deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27(2):120–39. https://doi.org/10.1080/10590500902885684.
Zhang X, Li XH, Ma X, Wang ZH, Lu S, Guo YL. Redox-induced apoptosis of human oocytes in resting follicles in vitro. J Soc Gynecol Investig. 2006;13(6):451–8. https://doi.org/10.1016/j.jsgi.2006.05.005.
Tamura H, Takasaki A, Miwa I, et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res. 2008;44(3):280–7. https://doi.org/10.1111/j.1600-079X.2007.00524.x.
Rodgers R, Carter J, Reid G, et al. The effect of laparoscopic salpingectomy for ectopic pregnancy on ovarian reserve. Aust N Z J Obstet Gynaecol. 2020;60(2):278–83. https://doi.org/10.1111/ajo.13129.
Eken MK, Ersoy GS, Kaygusuz EI, et al. Etanercept protects ovarian reserve against ischemia/reperfusion injury in a rat model. Arch Med Sci. 2019;15(4):1104–12. https://doi.org/10.5114/aoms.2017.72406.
Funding
This study was supported by the Ege University Scientific Research Projects Coordination Unit (grant number: TGA-2019–20808 (to Ilkben Gunusen)).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
Experimental procedures used in this study were approved by Ege University, Local Ethics Committee for Animal Experiments (approval no: 2019–045). All experimental procedures carried out in the same center with strict accordance with the recommendations in the ARRIVE guidelines (Animals in Research: Reporting In Vivo Experiments).
Conflict of Interest
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Gunusen, I., Akdemir, A., Gurel, C. et al. The Effects of Different Pressure Pneumoperitoneum Models Created By Standard or Heated-Humidified CO2 Insufflation on Ovary and Peritoneum: an Experimental Study in Rats. Reprod. Sci. 29, 1197–1208 (2022). https://doi.org/10.1007/s43032-022-00878-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-022-00878-2