Abstract
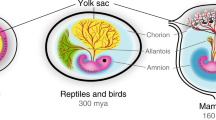
The yolk sac (YS) is the oldest of the extraembryonic membranes in vertebrates. Considered a transitory structure in the human species, the importance of the YS for a successful pregnancy is often overlooked. Due to the general inaccessibility of healthy human YS tissue for research, the use of experimental animal models is of great value. In order to better understand whether the mouse could be used as a translational model for the study of the human YS under normal and pathological conditions, this review comprehensively describes key developmental aspects of the human and mouse YS, detailing their development and function. YS major similarities in both species comprise the following: (1) histological composition (both being composed of endoderm, mesoderm, and mesothelium layers); (2) endoderm endocytosis, synthesis, secretion, and transport capabilities; and (3) mesoderm onset of haematopoiesis and angiogenesis. Examples of main dissimilarities include (1) persistence across pregnancy (i.e. early pregnancy in humans vs term pregnancy in mice); (2) the existence of a secondary YS in humans; (3) the presence of proliferative primordial germ cells (PGCs) in the human versus their absence in mice; and (4) eversion of histological layers in the mouse. Although these differences should be considered when interpreting data from mouse-based studies, the overall morphofunctional similarities in the YS between these species indicate that the mouse can be potentially used as a translational model for the study of the human YS.




Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Sheng G, Foley AC. Diversification and conservation of the extraembryonic tissues in mediating nutrient uptake during amniote development. Ann N Y Acad Sci. 2012;1271:97–103. https://doi.org/10.1111/j.1749-6632.2012.06726.x.
Ferner K, Mess A. Evolution and development of fetal membranes and placentation in amniote vertebrates. Respir Physiol Neurobiol. 2011;178:39–50. https://doi.org/10.1016/j.resp.2011.03.029.
Ross C, Boroviak TE. Origin and function of the yolk sac in primate embryogenesis. Nat Commun. 2020;11:3760. https://doi.org/10.1038/s41467-020-17575-w.
Mamsen LS, Brøchner CB, Byskov AG, Møllgard K. The migration and loss of human primordial germ stem cells from the hind gut epithelium towards the gonadal ridge. Int J Dev Biol. 2012;56:771–8. https://doi.org/10.1387/ijdb.120202lm.
Palis J. Yolk-sac hematopoiesis: the first blood cells of mouse and man. Exp Hematol. 2001;29:927–36. https://doi.org/10.1016/S0301-472X(01)00669-5.
McGrath KE, Palis J. Hematopoiesis in the yolk sac: more than meets the eye. Exp Hematol. 2005;33:1021–8. https://doi.org/10.1016/j.exphem.2005.06.012.
Golub R, Cumano A. Embryonic hematopoiesis. Blood Cells Mol Dis. 2013;51:226–31. https://doi.org/10.1016/j.bcmd.2013.08.004.
Berdahl DM, Blaine J, Van Voorhis B, Dokras A. Detection of enlarged yolk sac on early ultrasound is associated with adverse pregnancy outcomes. Fertil Steril. 2010;94:1535–7. https://doi.org/10.1016/j.fertnstert.2009.12.064.
Duan Y, Wang H, Mitchell-silbaugh K, Cai S, Fan F, Li Y, et al. Heat shock protein 60 regulates yolk sac erythropoiesis in mice. Cell Death Dis. 2019;10:766. https://doi.org/10.1038/s41419-019-2014-2.
Zohn IE, Sarkar AA. The visceral yolk sac endoderm provides for absorption of nutrients to the embryo during neurulation. Birt Defects Res A Clin Mol Teratol. 2010;88:593–600. https://doi.org/10.1002/bdra.20705.
Exalto N. Yolk sac abnormalities: a clinical review. In: Nogales FF, editor. Hum. Yolk sac yolk sac tumors, Berlin, Heidelberg: Springer Berlin Heidelberg; 1993, p. 126–34. https://doi.org/10.1007/978-3-642-77852-0_7.
Dong J, Wen L, Guo X, Xiao X, Jiang F, Li B, et al. The increased expression of glucose transporters in human full-term placentas from assisted reproductive technology without changes of mTOR signaling. Placenta. 2019;86:4–10. https://doi.org/10.1016/j.placenta.2019.08.087.
Nakamura T, Okamoto I, Sasaki K, Yabuta Y, Iwatani C, Tsuchiya H, et al. A developmental coordinate of pluripotency among mice, monkeys and humans. Nature. 2016;537:57–62. https://doi.org/10.1038/nature19096.
Bianchi DW, Wilkins-Haug LE, Enders AC, Hay ED. Origin of extraembryonic mesoderm in experimental animals: relevance to chorionic mosaicism in humans. Am J Med Genet. 1993;46:542–50. https://doi.org/10.1002/ajmg.1320460517.
Enders AC, King BF. Formation and differentiation of extraembryonic mesoderm in the rhesus monkey. Am J Anat. 1988;181:327–40. https://doi.org/10.1002/aja.1001810402.
Tan S, Pektaş MK, Arslan H. Sonographic evaluation of the yolk sac. J Ultrasound Med. 2012;31:87–95. https://doi.org/10.7863/jum.2012.31.1.87.
Freyer C, Renfree MB. The mammalian yolk sac placenta. J Exp Zoolog B Mol Dev Evol. 2009;312:545–54. https://doi.org/10.1002/jez.b.21239.
Jones CJ, Jauniaux E. Ultrastructure of the materno-embryonic interface in the first trimester of pregnancy. Micron Oxf Engl. 1993;1995(26):145–73. https://doi.org/10.1016/0968-4328(95)00002-l.
de Sousa Lopes SMC, Mummery CL. Differentiation in early development. Essent. Stem Cell Biol., Elsevier; 2014, p. 121–39. https://doi.org/10.1016/B978-0-12-409503-8.00010-X.
Carter AM. IFPA Senior Award Lecture: mammalian fetal membranes. Placenta. 2016;48:S21-30. https://doi.org/10.1016/j.placenta.2015.10.012.
Jauniaux E, Moscoso JG. Morphology and significance of the human yolk sac. In: Barnea ER, Hustin J, Jauniaux E, editors. First twelve weeks gestation, Berlin, Heidelberg: Springer Berlin Heidelberg; 1992, p. 192–213. https://doi.org/10.1007/978-3-642-84385-3_11.
Sauerbrei E, Cooperberg PL, Poland BJ. Ultrasound demonstration of the normal fetal yolk sac. J Clin Ultrasound. 1980;8:217–20. https://doi.org/10.1002/jcu.1870080306.
Crooij MJ, Westhuis M, Schoemaker J, Exalto N. Ultrasonographic measurement of the yolk sac. BJOG Int J Obstet Gynaecol. 1982;89:931–4. https://doi.org/10.1111/j.1471-0528.1982.tb05060.x.
Barzilai M, Lyons EA, Levi CS, Lindsay DJ. Vitelline duct cyst or double yolk sac. J Ultrasound Med. 1989;8:523–6. https://doi.org/10.7863/jum.1989.8.9.523.
Lindsay DJ, Lovett IS, Lyons EA, Levi CS, Zheng XH, Holt SC, et al. Yolk sac diameter and shape at endovaginal US: predictors of pregnancy outcome in the first trimester. Radiology. 1992;183:115–8. https://doi.org/10.1148/radiology.183.1.1549656.
Pereda J, Monge JI, Niimi G. Two different pathways for the transport of primitive and definitive blood cells from the yolk sac to the embryo in humans. Microsc Res Tech. 2010;73:803–9. https://doi.org/10.1002/jemt.20823.
Pereda J, Correr S, Motta PM. The structure of the human yolk sac: a scanning and transmission electron microscopic analysis. Arch Histol Cytol. 1994;57:107–17. https://doi.org/10.1679/aohc.57.107.
Cindrova-Davies T, Jauniaux E, Elliot MG, Gong S, Burton GJ, Charnock-Jones DS. RNA-seq reveals conservation of function among the yolk sacs of human, mouse, and chicken. Proc Natl Acad Sci. 2017;114:E4753–61. https://doi.org/10.1073/pnas.1702560114.
Pereda TJ, Motta PM. New advances in human embryology: morphofunctional relationship between the embryo and the yolk sac. Med Electron Microsc Off J Clin Electron Microsc Soc Jpn. 1999;32:67–78. https://doi.org/10.1007/s007950050011.
Jordan HE. A further study of the human umbilical vesicle. Anat Rec. 1910;4:341–53. https://doi.org/10.1002/ar.1090040903.
Tavian M, Peault B. The changing cellular environments of hematopoiesis in human development in utero. Exp Hematol. 2005;33:1062–9. https://doi.org/10.1016/j.exphem.2005.06.025.
Luckett WP. Origin and differentiation of the yolk sac and extraembryonic mesoderm in presomite human and rhesus monkey embryos. Am J Anat. 1978;152:59–97. https://doi.org/10.1002/aja.1001520106.
Bloom W, Bartelmez GW. Hematopoiesis in young human embryos. Am J Anat. 1940;67:21–53. https://doi.org/10.1002/aja.1000670103.
Takashina T. Histology of the secondary human yolk sac with special reference to hematopoiesis. In: Nogales FF, editor. Hum. Yolk sac yolk sac tumors, Berlin, Heidelberg: Springer Berlin Heidelberg; 1993, p. 48–69. https://doi.org/10.1007/978-3-642-77852-0_3.
Ivanovs A, Rybtsov S, Ng ES, Stanley EG, Elefanty AG, Medvinsky A. Human haematopoietic stem cell development: from the embryo to the dish. Development. 2017;144:2323–37. https://doi.org/10.1242/dev.134866.
Tavian M, Hallais MF, Péault B. Emergence of intraembryonic hematopoietic precursors in the pre-liver human embryo. Dev Camb Engl. 1999;126:793–803.
Reece EA, Pinter E, Naftolin F. Experimental models of injury in the mammalian yolk sac. In: Nogales FF, editor. Hum. Yolk sac yolk sac tumors, Berlin, Heidelberg: Springer Berlin Heidelberg; 1993, p. 135–60. https://doi.org/10.1007/978-3-642-77852-0_8.
Fuss A. Über die Geschlechtszellen des Menschen und der Säugetiere. Arch Für Mikrosk Anat. 1912;81:a1-23. https://doi.org/10.1007/BF02980550.
De Felici M. Origin, migration, and proliferation of human primordial germ cells. In: Coticchio G, Albertini DF, De Santis L, editors. Oogenesis, London: Springer London; 2013, p. 19–37. https://doi.org/10.1007/978-0-85729-826-3_2.
Carter AM, Enders AC. Placentation in mammals: definitive placenta, yolk sac, and paraplacenta. Theriogenology. 2016;86:278–87. https://doi.org/10.1016/j.theriogenology.2016.04.041.
Pereda J, Niimi G. Embryonic erythropoiesis in human yolk sac: two different compartments for two different processes. Microsc Res Tech. 2008;71:856–62. https://doi.org/10.1002/jemt.20627.
Hesseldahl H, Larsen JF. Ultrastructure of human yolk sac: endoderm, mesenchyme, tubules and mesothelium. Am J Anat. 1969;126:315–35. https://doi.org/10.1002/aja.1001260306.
Gitlin D, Perricelli A. Synthesis of serum albumin, prealbumin, α-foetoprotein, α1-antitrypsin and transferrin by the human yolk sac. Nature. 1970;228:995–7. https://doi.org/10.1038/228995a0.
Shi WK, Hopkins B, Thompson S, Heath JK, Luke BM, Graham CF. Synthesis of apolipoproteins, alphafoetoprotein, albumin, and transferrin by the human foetal yolk sack and other foetal organs. J Embryol Exp Morphol. 1985;85:191–206.
Gulbis B, Jauniaux E, Jurkovic D, Thiry P, Campbell S, Ooms HA. Determination of protein pattern in embryonic cavities of human early pregnancies: a means to understand materno-embryonic exchanges. Hum Reprod. 1992;7:886–9. https://doi.org/10.1093/oxfordjournals.humrep.a137755.
Buffe D, Rimbaut C, Gaillard JA. α-Fetoprotein and other proteins in the human yolk sac. In: Nogales FF, editor. Hum. Yolk sac yolk sac tumors, Berlin, Heidelberg: Springer Berlin Heidelberg; 1993, p. 109–25. https://doi.org/10.1007/978-3-642-77852-0_6.
Murray SA, Morgan JL, Kane C, Sharma Y, Heffner CS, Lake J, et al. Mouse gestation length is genetically determined. PLoS ONE. 2010;5: e12418. https://doi.org/10.1371/journal.pone.0012418.
Jollie WP. Development, morphology, and function of the yolk-sac placenta of laboratory rodents. Teratology. 1990;41:361–81. https://doi.org/10.1002/tera.1420410403.
Hafez S. Comparative placental anatomy. Prog. Mol. Biol. Transl. Sci., vol. 145, Elsevier; 2017, p. 1–28. https://doi.org/10.1016/bs.pmbts.2016.12.001.
Beckman DA, Koszalka TR, Jensen M, Brent RL. Experimental manipulation of the rodent visceral yolk sac. Teratology. 1990;41:395–404. https://doi.org/10.1002/tera.1420410405.
Bevilacqua E, Lorenzon AR, Bandeira CL, Hoshida MS. Biology of the ectoplacental cone. Guide Investig. Mouse Pregnancy, Elsevier; 2014, p. 113–24. https://doi.org/10.1016/B978-0-12-394445-0.00010-2.
Carter AM, Pijnenborg R. Emil Selenka on the embryonic membranes of the mouse and placentation in gibbons and orangutans. Placenta. 2016;37:65–71. https://doi.org/10.1016/j.placenta.2015.11.005.
Dobreva MP, Lhoest L, Pereira PNG, Umans L, Camus A, Chuva de Sousa Lopes SM, et al. Periostin as a biomarker of the amniotic membrane. Stem Cells Int 2012;2012:987185. https://doi.org/10.1155/2012/987185.
Pereira PN, Dobreva MP, Graham L, Huylebroeck D, Lawson KA, Zwijsen A. Amnion formation in the mouse embryo: the single amniochorionic fold model. BMC Dev Biol. 2011;11:48. https://doi.org/10.1186/1471-213X-11-48.
Dobreva MP, Pereira PNG, Deprest J, Zwijsen A. On the origin of amniotic stem cells: of mice and men. Int J Dev Biol. 2010;54:761–77. https://doi.org/10.1387/ijdb.092935md.
King BF, Enders AC. Comparative development of the mammalian yolk sac. In: Nogales FF, editor. Hum. Yolk sac yolk sac tumors, Berlin, Heidelberg: Springer Berlin Heidelberg; 1993, p. 1–32. https://doi.org/10.1007/978-3-642-77852-0_1.
Niimi G, Usuda N, Shinzato M, Nagamura Y. A light and electron microscopic study of the mouse visceral yolk sac endodermal cells in the middle and late embryonic periods, showing the possibility of definitive erythropoiesis. Ann Anat - Anat Anz. 2002;184:425–9. https://doi.org/10.1016/S0940-9602(02)80073-5.
Zon L. Developmental biology of hematopoiesis. Blood. 1995;86:2876–91. https://doi.org/10.1182/blood.V86.8.2876.2876.
Yamane T. Mouse yolk sac hematopoiesis. Front Cell Dev Biol. 2018;6:80. https://doi.org/10.3389/fcell.2018.00080.
Tober J, Koniski A, McGrath KE, Vemishetti R, Emerson R, de Mesy-Bentley KKL, et al. The megakaryocyte lineage originates from hemangioblast precursors and is an integral component both of primitive and of definitive hematopoiesis. Blood. 2007;109:1433–41. https://doi.org/10.1182/blood-2006-06-031898.
Palis J, Robertson S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Dev Camb Engl. 1999;126:5073–84.
Takahashi K, Yamamura F, Naito M. Differentiation, maturation, and proliferation of macrophages in the mouse yolk sac: a light-microscopic, enzyme-cytochemical, immunohistochemical, and ultrastructural study. J Leukoc Biol. 1989;45:87–96. https://doi.org/10.1002/jlb.45.2.87.
Yamane T. Cellular Basis of Embryonic Hematopoiesis and its implications in prenatal erythropoiesis. Int J Mol Sci. 2020;21:9346. https://doi.org/10.3390/ijms21249346.
Jaffredo T, Nottingham W, Liddiard K, Bollerot K, Pouget C, Debruijn M. From hemangioblast to hematopoietic stem cell: an endothelial connection? Exp Hematol. 2005;33:1029–40. https://doi.org/10.1016/j.exphem.2005.06.005.
Neo WH, Meng Y, Rodriguez-Meira A, Fadlullah MZH, Booth CAG, Azzoni E, et al. Ezh2 is essential for the generation of functional yolk sac derived erythro-myeloid progenitors. Nat Commun. 2021;12:7019. https://doi.org/10.1038/s41467-021-27140-8.
Brent RL, Beckman DA, Jensen M, Koszalka TR. Experimental yolk sac dysfunction as a model for studying nutritional disturbances in the embryo during early organogenesis. Teratology. 1990;41:405–13. https://doi.org/10.1002/tera.1420410406.
Farese RV, Ruland SL, Flynn LM, Stokowski RP, Young SG. Knockout of the mouse apolipoprotein B gene results in embryonic lethality in homozygotes and protection against diet-induced hypercholesterolemia in heterozygotes. Proc Natl Acad Sci. 1995;92:1774–8. https://doi.org/10.1073/pnas.92.5.1774.
Bielinska M, Narita N, Wilson DB. Distinct roles for visceral endoderm during embryonic mouse development. Int J Dev Biol. 1999;43:183–205.
Terasawa Y, Cases SJ, Wong JS, Jamil H, Jothi S, Traber MG, et al. Apolipoprotein B-related gene expression and ultrastructural characteristics of lipoprotein secretion in mouse yolk sac during embryonic development. J Lipid Res. 1999;40:1967–77.
Ekblom P, Thesleff I. Control of kidney differentiation by soluble factors secreted by the embryonic liver and the yolk sac. Dev Biol. 1985;110:29–38. https://doi.org/10.1016/0012-1606(85)90060-0.
Salbaum JM, Finnell RH, Kappen C. Regulation of folate receptor 1 gene expression in the visceral endoderm. Birt Defects Res A Clin Mol Teratol. 2009;85:303–13. https://doi.org/10.1002/bdra.20537.
Seo MJ, Lim JH, Kim D-H, Bae H-R. Loss of aquaporin-3 in placenta and fetal membranes induces growth restriction in mice. Dev Reprod. 2018;22:263–73. https://doi.org/10.12717/DR.2018.22.3.263.
Kim J, Mohanty S, Ganesan LP, Hua K, Jarjoura D, Hayton WL, et al. FcRn in the yolk sac endoderm of mouse is required for IgG transport to fetus. J Immunol. 2009;182:2583–9. https://doi.org/10.4049/jimmunol.0803247.
Burton GJ, Hempstock J, Jauniaux E. Nutrition of the human fetus during the first trimester—a review. Placenta. 2001;22:S70–7. https://doi.org/10.1053/plac.2001.0639.
Campbell J, Wathen N, Perry G, Soneji S, Sourial N, Chard T. The coelomic cavity: an important site of materno-fetal nutrient exchange in the first trimester of pregnancy. BJOG Int J Obstet Gynaecol. 1993;100:765–7. https://doi.org/10.1111/j.1471-0528.1993.tb14271.x.
Bloise E, Ortiga-Carvalho TM, Reis FM, Lye SJ, Gibb W, Matthews SG. ATP-binding cassette transporters in reproduction: a new frontier. Hum Reprod Update 2015:dmv049. https://doi.org/10.1093/humupd/dmv049.
Imperio GE, Javam M, Lye P, Constantinof A, Dunk CE, Reis FM, et al. Gestational age-dependent gene expression profiling of ATP-binding cassette transporters in the healthy human placenta. J Cell Mol Med. 2019;23:610–8. https://doi.org/10.1111/jcmm.13966.
Fontes KN, Reginatto MW, Silva NL, Andrade CBV, Bloise FF, Monteiro VRS, et al. Dysregulation of placental ABC transporters in a murine model of malaria-induced preterm labor. Sci Rep. 2019;9:11488. https://doi.org/10.1038/s41598-019-47865-3.
Eustaquio Do Imperio G, Lye P, Bloise E, Matthews SG. Function of multidrug resistance transporters is disrupted by infection mimics in human brain endothelial cells. Tissue Barriers. 2021;9:1860616. https://doi.org/10.1080/21688370.2020.1860616.
Bloise E, Matthews SG. Multidrug resistance P-glycoprotein (P-gp), glucocorticoids, and the stress response. Stress Physiol. Biochem. Pathol., Elsevier; 2019, p. 227–41. https://doi.org/10.1016/B978-0-12-813146-6.00019-9.
Martinelli LM, Fontes KN, Reginatto MW, Andrade CBV, Monteiro VRS, Gomes HR, et al. Malaria in pregnancy regulates P-glycoprotein (P-gp/Abcb1a) and ABCA1 efflux transporters in the mouse visceral yolk sac. J Cell Mol Med. 2020;24:10636–47. https://doi.org/10.1111/jcmm.15682.
Martinelli LM, Reginatto MW, Fontes KN, Andrade CBV, Monteiro VRS, Gomes HR, et al. Breast cancer resistance protein (Bcrp/Abcg2) is selectively modulated by lipopolysaccharide (LPS) in the mouse yolk sac. Reprod Toxicol. 2020;98:82–91. https://doi.org/10.1016/j.reprotox.2020.09.001.
Bloise E, Bhuiyan M, Audette MC, Petropoulos S, Javam M, Gibb W, et al. Prenatal endotoxemia and placental drug transport in the mouse: placental size-specific effects. PLoS ONE. 2013;8: e65728. https://doi.org/10.1371/journal.pone.0065728.
Reginatto MW, Fontes KN, Monteiro VRS, Silva NL, Andrade CBV, Gomes HR, et al. Effect of sublethal prenatal endotoxaemia on murine placental transport systems and lipid homeostasis. Front Microbiol. 2021;12: 706499. https://doi.org/10.3389/fmicb.2021.706499.
Brent RL, Fawcett LB. Nutritional studies of the embryo during early organogenesis with normal embryos and embryos exhibiting yolk sac dysfunction. J Pediatr. 1998;132:S6-16. https://doi.org/10.1016/S0022-3476(98)70522-0.
Mony VK, Benjamin S, O’Rourke EJ. A lysosome-centered view of nutrient homeostasis. Autophagy. 2016;12:619–31. https://doi.org/10.1080/15548627.2016.1147671.
Jones CJP, Choudhury RH, Aplin JD. Tracking nutrient transfer at the human maternofetal interface from 4 weeks to term. Placenta. 2015;36:372–80. https://doi.org/10.1016/j.placenta.2015.01.002.
Saitou M, Yamaji M. Primordial germ cells in mice. 2012;4. https://doi.org/10.1101/cshperspect.a008375.
Funding
This work was supported by funding from Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES, finance Code 001) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, 310578/2020–5).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All authors have stated for consent of publication.
Conflict of Interest
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Martinelli, L.M., Carucci, A., Payano, V.J.H. et al. Translational Comparison of the Human and Mouse Yolk Sac Development and Function. Reprod. Sci. 30, 41–53 (2023). https://doi.org/10.1007/s43032-022-00872-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-022-00872-8