Abstract
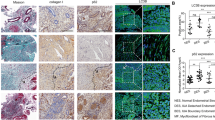
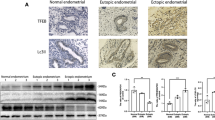
Uterine fibroids (UF) represent an immense health burden throughout the world. Obesity is considered one of the risk factors for UF development; however, the underlying mechanisms remain largely unexplored. We investigated the effect of obesity on fibroblast activation and its association with inflammation, autophagy dysfunction, and oxidative stress in UF patients. Thirty-five pre-menopausal UF patients were included in this study and classified into non-obese group (BM1 ≤ 30 kg/m2, n = 15) and obese group (BMI > 30 kg/m2, n = 20). Tissue samples were collected from fibroids and adjacent normal myometrium. Our results showed increased expression of fibroblast activation protein (FAP) together with markers of autophagy, inflammation, and oxidative stress in UF patients, which were all more markedly upregulated in obese compared to non-obese patients. In addition, BMI was significantly positive correlated with FAP and autophagy markers. In conclusion, the results of the present study suggest that obesity-associated autophagy dysregulation together with increased FAP expression may increase the risk of UFs in obese women.






Similar content being viewed by others
Availability of Data and Materials
All related data and materials are available from the corresponding author upon request.
Code Availability
Non applicable.
References
De La Cruz MSD, Buchanan EM. Uterine fibroids: diagnosis and treatment. Am Fam Physician. 2017;95(2):100–7.
Giuliani E, As-Sanie S, Marsh EE. Epidemiology and management of uterine fibroids. Int J Gynecol Obstet. 2020;149(1):3–9.
Marsh EE, Al-Hendy A, Kappus D, Galitsky A, Stewart EA, Kerolous M. Burden, Prevalence, and treatment of uterine fibroids: a survey of US women. J Women’s Health. 2018;27(11):1359–67.
Fortin C, Flyckt R, Falcone T. Alternatives to hysterectomy: the burden of fibroids and the quality of life. Best Pract Res Clin Obstet Gynaecol. 2018;46:31–42.
Zhao R, Wang X, Zou L, Li G, Chen Y, Li C, et al. Adverse obstetric outcomes in pregnant women with uterine fibroids in China: a multicenter survey involving 112,403 deliveries. Plos One. 2017;12(11):e0187821.
Garg D, Segars JH. Treatment modalities for fibroids, indications, risks, and benefits. In: Moawad N, editor. Uterine Fibroids. Cham: Springer; 2018. p. 87–106.
Pandey S, Bhattacharya S. Impact of obesity on gynecology. Womens Health. 2010;6:107–17.
Okolo S. Incidence, aetiology and epidemiology of uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22(4):571–88.
Templeman C, Marshall SF, Clarke CA, DeLellis HK, Largent J, Neuhausen S, et al. Risk factors for surgically removed fibroids in a large cohort of teachers. Fertil Steril. 2009;92(4):1436–46.
Wise LA, Laughlin-Tommaso SK. Epidemiology of uterine fibroids–from menarche to menopause. Clin Obstet Gynecol. 2016;59(1):2–24.
Soave I, Marci R. From obesity to uterine fibroids: an intricate network. Curr Med Res Opin. 2018;34(11):1877–9.
Ciavattini A, Delli Carpini G, Moriconi L, Clemente N, Orici F, Boschi AC, et al. The association between ultrasound-estimated visceral fat deposition and uterine fibroids: an observational study. Gynecol Endocrinol. 2017;33(8):634–7.
Del Bello B, Marcolongo P, Ciarmela P, Sorbi F, Petraglia F, Luisi S, et al. Autophagy up-regulation by ulipristal acetate as a novel target mechanism in the treatment of uterine leiomyoma: an in vitro study. Fertil Steril. 2019;112(6):1150–9.
Guo J, Zheng L, Chen L, Luo N, Yang W, Qu X, et al. Lipopolysaccharide activated TLR4/NF-κB signaling pathway of fibroblasts from uterine fibroids. Int J Clin Exp Pathol. 2015;8(9):10014–25.
Lin W, Ma S, Wang L. Study on the correlation of MLCK and FAP expression with uterine fibroid cell proliferation and invasion. J Hainan Med Univ. 2017;23(12):79–82.
Errarte P, Guarch R, Pulido R, Blanco L, Nunes-Xavier CE, Beitia M, et al. The expression of fibroblast activation protein in clear cell renal cell carcinomas is associated with synchronous lymph node metastases. Plos One. 2016;11(12):e0169105.
Gao L-M, Wang F, Zheng Y, Fu Z-Z, Zheng L, Chen L-L. Roles of fibroblast activation protein and hepatocyte growth factor expressions in angiogenesis and metastasis of gastric cancer. Pathol Oncol Res. 2019;25(1):369–76.
Zhang HE, Hamson EJ, Koczorowska MM, Tholen S, Chowdhury S, Bailey CG, et al. Identification of novel natural substrates of fibroblast activation protein-alpha by differential degradomics and proteomics. Mol Cell Proteom. 2019;18(1):65–85.
Luo N, Guan Q, Zheng L, Qu X, Dai H, Cheng Z. Estrogen-mediated activation of fibroblasts and its effects on the fibroid cell proliferation. Transl Res. 2014;163(3):232–41.
Hewitt G, Korolchuk VI. Repair, reuse, recycle: the expanding role of autophagy in genome maintenance. Trends Cell Biol. 2017;27(5):340–51.
El Andaloussi A, Habib S, Soylemes G, Laknaur A, Elhusseini H, Al-Hendy A, et al. Defective expression of ATG4D abrogates autophagy and promotes growth in human uterine fibroids. Cell Death Discov. 2017;3(1):1–9.
Namkoong S, Cho CS, Semple I, Lee JH. Autophagy dysregulation and obesity-associated pathologies. Mol Cells. 2018;41(1):3–10.
Zhang Y, Sowers JR, Ren J. Targeting autophagy in obesity: from pathophysiology to management. Nat Rev Endocrinol. 2018;14(6):356–76.
Castañeda D, Gabani M, Choi S-K, Nguyen QM, Chen C, Mapara A, et al. Targeting autophagy in obesity-associated heart disease. Obesity. 2019;27(7):1050–8.
Filomeni G, De Zio D, Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ. 2015;22(3):377–88.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25(4):402–8.
Bancroft JD, Gamble M. Theory and practice of histological techniques. 6th ed. China: Churchill Livingstone, Elsevier; 2008.
Glauert AM, Lewis PR. Biological specimen preparation for transmission electron microscopy. In: Glauert AM, editor. Practical methods in electron microscopy. London: Portland Press; 1998.
Blomberg R, Beiting DP, Wabitsch M, Puré E. Fibroblast activation protein restrains adipogenic differentiation and regulates matrix-mediated mTOR signaling. Matrix Biol. 2019;83:60–76.
Qin H, Lin Z, Vásquez E, Luan X, Guo F, Xu L. Association between obesity and the risk of uterine fibroids: a systematic review and meta-analysis. J Epidemiol Community Health. 2020;75(2):197–204.
Çinar M, Tokmak A, Güzel AI, Aksoy RT, Özer İ, Yilmaz N, et al. Association of clinical outcomes and complications with obesity in patients who have undergone abdominal myomectomy. J Chin Med Assoc. 2016;79(8):435–9.
Marshall LM, Spiegelman D, Manson JE, Goldman MB, Barbieri RL, Stampfer MJ, et al. Risk of uterine leiomyomata among premenopausal women in relation to body size and cigarette smoking. J Epidemiol. 1998;9(5):511–7.
Sato F, Nishi M, Kudo R, Miyake H. Body fat distribution and uterine leiomyomas. J Epidemiol. 1998;8(3):176–80.
Poret JM, Souza-Smith F, Marcell SJ, Gaudet DA, Tzeng TH, Braymer HD, et al. High fat diet consumption differentially affects adipose tissue inflammation and adipocyte size in obesity-prone and obesity-resistant rats. Int J Obes. 2018;42(3):535–41.
Protic O, Toti P, Islam MS, Occhini R, Giannubilo SR, Catherino WH, et al. Possible involvement of inflammatory/reparative processes in the development of uterine fibroids. Cell Tissue Res. 2016;364(2):415–27.
Winkler G, Kiss S, Keszthelyi L, Sápi Z, Ory I, Salamon F, et al. Expression of tumor necrosis factor (TNF)-a protein in the subcutaneous and visceral adipose tissue in correlation with adipocyte cell volume, serum TNF-a, soluble serum TNF-receptor-2 concentrations and C-peptide level. Eur J Endocrinol. 2003;149(2):129–35.
Petrus P, Mejhert N, Corrales P, Lecoutre S, Li Q, Maldonado E, et al. Transforming growth factor-β3 regulates adipocyte number in subcutaneous white adipose tissue. Cell Rep. 2018;25(3):551–60.
Ihara S, Hirata Y, Koike K. TGF-β in inflammatory bowel disease: a key regulator of immune cells, epithelium, and the intestinal microbiota. J Gastroenterol. 2017;52(7):777–87.
Pervin S, Nyah W, Reddy ST, Singh R. Novel aspects of follistatin/transforming growth factor-β (TGF-β) signaling in adipose tissue metabolism: implications in metabolic health. In: Szablewski L, editor. Adipose Tissue-An Update. London: IntechOpen; 2019. p. 1–21. https://doi.org/10.5772/intechopen.88294.
Arici A, Sozen I. Transforming growth factor-β3 is expressed at high levels in leiomyoma where it stimulates fibronectin expression and cell proliferation. Fertil Steril. 2000;73(5):1006–11.
Lee BS, Nowak RA. Human leiomyoma smooth muscle cells show increased expression of transforming growth factor-β3 (TGFβ3) and altered responses to the antiproliferative effects of TGFβ. J Clin Endocrinol Metab. 2001;86(2):913–20.
Plewka A, Madej P, Plewka D, Kowalczyk A, Miskiewicz A, Wittek P, et al. Immunohistochemical localization of selected pro-inflammatory factors in uterine myomas and myometrium in women of various ages. Folia Histochem Cytobiol. 2013;51(1):73–83.
Tal R, Segars JH. The role of angiogenic factors in fibroid pathogenesis: potential implications for future therapy. Hum Reprod Update. 2014;20(2):194–216.
Fletcher N, Saed M, Abu-Soud H, Al-Hendy A, Diamond MP, Saed G. Uterine fibroids are characterized by an impaired antioxidant cellular system: potential role of hypoxia in the pathophysiology of uterine fibroids. J Assist Reprod Genet. 2013;30(7):969–74.
Chiou J-F, Hu M-L. Elevated lipid peroxidation and disturbed antioxidant enzyme activities in plasma and erythrocytes of patients with uterine cervicitis and myoma. Clin Biochem. 1999;32(3):189–92.
Pejic S, Kasapovic J, Todorovic A, Stojiljkovic V, Pajovic SB. Lipid peroxidation and antioxidant status in blood of patients with uterine myoma, endometrial polypus, hyperplastic and malignant endometrium. Biol Res. 2006;39(4):619–29.
Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;13(4):851–63.
Islam MS, Ciavattini A, Petraglia F, Castellucci M, Ciarmela P. Extracellular matrix in uterine leiomyoma pathogenesis: a potential target for future therapeutics. Hum Reprod Update. 2018;24(1):59–85.
Bao H, Sin TK, Zhang G. Activin A induces leiomyoma cell proliferation, extracellular matrix (ECM) accumulation and myofibroblastic transformation of myometrial cells via p38 MAPK. Biochem Biophys Res Commun. 2018;504(2):447–53.
Ciebiera M, Włodarczyk M, Zgliczyńska M, Łukaszuk K, Męczekalski B, Kobierzycki C, et al. The role of tumor necrosis factor α in the biology of uterine fibroids and the related symptoms. Int J Mol Sci. 2018;19(12):3869–95.
Borahay MA, Asoglu MR, Mas A, Adam S, Kilic GS, Al-Hendy A. Estrogen receptors and signaling in fibroids: role in pathobiology and therapeutic implications. Reprod Sci. 2017;24(9):1235–44.
Le Goff C, Cormier-Daire V. From tall to short: the role of TGFβ signaling in growth and its disorders. Am J Med Genet. 2012;160(3):145–53.
Brokopp CE, Schoenauer R, Richards P, Bauer S, Lohmann C, Emmert MY, et al. Fibroblast activation protein is induced by inflammation and degrades type I collagen in thin-cap fibroatheromata. Eur Heart J. 2011;32(21):2713–22.
Joseph DS, Malik M, Nurudeen S, Catherino WH. Myometrial cells undergo fibrotic transformation under the influence of transforming growth factor β-3. Fertil Steril. 2010;93(5):1500–8.
Kota A, Deshpande DA, Mehra Haghi BO, Sharma P. Autophagy and airway fibrosis: is there a link? F1000Res. 2017;6:409.
Donnez J, Tomaszewski J, Vázquez F, Bouchard P, Lemieszczuk B, Baró F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421–32.
Dikic I, Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19(6):349–64.
Komatsu M, Kageyama S, Ichimura Y. p62/SQSTM1/A170: physiology and pathology. Pharmacol Res. 2012;66(6):457–62.
Herder C, Schneitler S, Rathmann W, Haastert B, Schneitler H, Winkler H, et al. Low-grade inflammation, obesity, and insulin resistance in adolescents. J Clin Endocrinol Metab. 2007;92(12):4569–74.
Tripathi S, Srivastava S, Tripathi YB. Obesity and its complications: role of autophagy. Int J Pharm Sci Res. 2018;9(8):3100–13.
Ju L, Han J, Zhang X, Deng Y, Yan H, Wang C, et al. Obesity-associated inflammation triggers an autophagy–lysosomal response in adipocytes and causes degradation of perilipin 1. Cell Death Dis. 2019;10(2):1–16.
Najafi S, Abo-Ali EM, Dukhande VV. Methods for studying TNFα-induced autophagy. Methods Mol Biol. 2020;2108:131–46.
Tang Z, Hu B, Zang F, Wang J, Zhang X, Chen H. Nrf2 drives oxidative stress-induced autophagy in nucleus pulposus cells via a Keap1/Nrf2/p62 feedback loop to protect intervertebral disc from degeneration. Cell Death Dis. 2019;10(7):1–12.
Costa A, Scholer-Dahirel A, Mechta-Grigoriou F. The role of reactive oxygen species and metabolism on cancer cells and their microenvironment. Semin Cancer Biol. 2014;25:23–32.
Jain M, Rivera S, Monclus EA, Synenki L, Zirk A, Eisenbart J, et al. Mitochondrial reactive oxygen species regulate transforming growth factor-β signaling. J Biol Chem. 2013;288(2):770–7.
Sharma LK, Tiwari M, Rai NK, Bai Y. Mitophagy activation repairs Leber’s hereditary optic neuropathy-associated mitochondrial dysfunction and improves cell survival. Hum Mol Genet. 2019;28(3):422–33.
Dunshee DR, Bainbridge TW, Kljavin NM, Zavala-Solorio J, Schroeder AC, Chan R, et al. Fibroblast activation protein cleaves and inactivates fibroblast growth factor 21. J Biol Chem. 2016;291(11):5986–96.
Zhen EY, Jin Z, Ackermann BL, Thomas MK, Gutierrez JA. Circulating FGF21 proteolytic processing mediated by fibroblast activation protein. Biochem J. 2016;473(5):605–14.
Wu Y, Shi T, Wang J, He R. Talabostat alleviates obesity and associated metabolic dysfunction via suppression of macrophage-driven adipose inflammation. Obesity. 2021;29(2):327–36.
Funding
This research received a grant from the Assiut Medical School Grant Office. Grant no 2018-02-27-006-R1.
Author information
Authors and Affiliations
Contributions
Eman Radwan and Nashwa Maghraby: conceptualization, methodology, software, data curation, investigation, formal analysis, writing, visualization, and supervision; Nashwa A.M. Mostafa: methodology, investigation, formal analysis, visualization; Heba E.M El-Deek: methodology, investigation, formal analysis; Amira El-Noweihi and Nagla T. El-Melegy: conceptualization, visualization writing–reviewing, and editing; Ahmed M. Abbas: data curation, investigation, and formal analysis.
Corresponding author
Ethics declarations
Ethics Approval
All study procedures were approved by the Medical Ethics Committee, Faculty of Medicine, and Assiut University (IRB no: 17200173).
Consent to Participate
A written informed consent was obtained from each participant.
Consent for Publication
Participants have consented to the submission of data.
Conflict of Interest
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Maghraby, N., El Noweihi, A.M., El-Melegy, N.T. et al. Increased Expression of Fibroblast Activation Protein is Associated with Autophagy Dysregulation and Oxidative Stress in Obese Women with Uterine Fibroids. Reprod. Sci. 29, 448–459 (2022). https://doi.org/10.1007/s43032-021-00810-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00810-0