Abstract
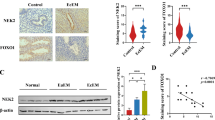
Decidualization is a substantive differentiation process experienced by endometrium to prepare for pregnancy. During this process, the endometrial stromal cells are transformed to endometrial epithelial cells. The receptivity of endometrium is necessary for the decidualization and successful implantation of endometrium, while the main hormones coordinating this process are estrogen and progesterone (P). In our study, the immunofluorescence, qPCR, and western blot experiments were conducted on different types of clinical endometrial tissue samples. The experimental results show that in the endometrium of normal subjects during the luteal phase, the protein level and serum P4 level of the orphan nuclear receptor NR4A1 messenger RNA were all significantly higher than those of patients with endometriosis or primary infertility, and the two levels presented positive correlation. Through decidualization induction of the human endometrial stromal cells (hESCs) cultured in vitro and additional P treatment, the results of chromatin immunoprecipitation and other experiments show that the P treatment could upregulate the expression of NR4A1 in hESCs, and this process was mediated under the direct effect of progesterone receptor (PR) and NR4A1. When the NR4A1 in hESCs was silenced, the promotion of hESC proliferation by P was inhibited. P and overexpressed NR4A1 increased the expression of epithelial cell marker in decidual hESCs, and qPCR showed that NR4A1’s response to P was earlier than that of the epithelial cell marker. The results of spheroid adhesion assay show that the silent NR4A1 had reduced the adhesion of decidual hESCs induced in vitro to embryo. To sum it up, NR4A1 participated in the decidualization process by responding to the P regulation via and by promoting the hESCs’ mesenchymal–epithelial transition, so as to further influence the receptivity of endometrium.






Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, et al. Molecular cues to implantation. Endocr Rev. 2004;25(3):341–73. https://doi.org/10.1210/er.2003-0020.
Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7(3):185–99. https://doi.org/10.1038/nrg1808.
Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, et al. Embryo implantation. Dev Biol. 2000;223(2):217–37. https://doi.org/10.1006/dbio.2000.9767.
Gellersen B, Brosens IA, Brosens JJ. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med. 2007;25(6):445–53. https://doi.org/10.1055/s-2007-991042.
Gellersen B, Brosens JJ. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev. 2014;35(6):851–905. https://doi.org/10.1210/er.2014-1045.
Simon C, Martin JC, Pellicer A. Paracrine regulators of implantation. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14(5):815–26. https://doi.org/10.1053/beog.2000.0121.
Aplin JD. The cell biological basis of human implantation. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14(5):757–64. https://doi.org/10.1053/beog.2000.0116.
Fox C, Morin S, Jeong JW, Scott RT Jr, Lessey BA. Local and systemic factors and implantation: what is the evidence? Fertil Steril. 2016;105(4):873–84. https://doi.org/10.1016/j.fertnstert.2016.02.018.
Lee KY, DeMayo FJ. Animal models of implantation. Reproduction. 2004;128(6):679–95. https://doi.org/10.1530/rep.1.00340.
Maruyama T, Yoshimura Y. Molecular and cellular mechanisms for differentiation and regeneration of the uterine endometrium. Endocr J. 2008;55(5):795–810. https://doi.org/10.1507/endocrj.k08e-067.
Yu L, Hu R, Sullivan C, Swanson RJ, Oehninger S, Sun YP, et al. MFGE8 regulates TGF-beta-induced epithelial mesenchymal transition in endometrial epithelial cells in vitro. Reproduction. 2016;152(3):225–33. https://doi.org/10.1530/REP-15-0585.
Cornillie FJ, Vasquez G, Brosens I. The response of human endometriotic implants to the anti-progesterone steroid R 2323: a histologic and ultrastructural study. Pathol Res Pract. 1985;180(6):647–55. https://doi.org/10.1016/S0344-0338(85)80044-3.
Tang M, Mazella J, Zhu HH, Tseng L. Ligand activated relaxin receptor increases the transcription of IGFBP-1 and prolactin in human decidual and endometrial stromal cells. Mol Hum Reprod. 2005;11(4):237–43. https://doi.org/10.1093/molehr/gah149.
Ramathal CY, Bagchi IC, Taylor RN, Bagchi MK. Endometrial decidualization: of mice and men. Semin Reprod Med. 2010;28(1):17–26. https://doi.org/10.1055/s-0029-1242989.
Strauss JF, Barbieri RL. Yen and Jaffe’s reproductive endocrinology physiology, pathophysiology, and clinical management. Elsevier; 2014.
Goddard LM, Murphy TJ, Org T, Enciso JM, Hashimoto-Partyka MK, Warren CM, et al. Progesterone receptor in the vascular endothelium triggers physiological uterine permeability preimplantation. Cell. 2014;156(3):549–62. https://doi.org/10.1016/j.cell.2013.12.025.
Palumbo-Zerr K, Zerr P, Distler A, Fliehr J, Mancuso R, Huang J, et al. Orphan nuclear receptor NR4A1 regulates transforming growth factor-beta signaling and fibrosis. Nat Med. 2015;21(2):150–8. https://doi.org/10.1038/nm.3777.
Wu X, Fu S, Liu Y, Luo H, Li F, Wang Y, et al. NDP-MSH binding melanocortin-1 receptor ameliorates neuroinflammation and BBB disruption through CREB/Nr4a1/NF-kappaB pathway after intracerebral hemorrhage in mice. J Neuroinflammation. 2019;16(1):192. https://doi.org/10.1186/s12974-019-1591-4.
Krikun G, Mor G, Alvero A, Guller S, Schatz F, Sapi E, et al. A novel immortalized human endometrial stromal cell line with normal progestational response. Endocrinology. 2004;145(5):2291–6. https://doi.org/10.1210/en.2003-1606.
Greening DW, Nguyen HP, Elgass K, Simpson RJ, Salamonsen LA. Human endometrial exosomes contain hormone-specific cargo modulating trophoblast adhesive capacity: insights into endometrial-embryo interactions. Biol Reprod. 2016;94(2):38. https://doi.org/10.1095/biolreprod.115.134890.
Zuo RJ, Gu XW, Qi QR, Wang TS, Zhao XY, Liu JL, et al. Warburg-like glycolysis and lactate shuttle in mouse decidua during early pregnancy. J Biol Chem. 2015;290(35):21280–91. https://doi.org/10.1074/jbc.M115.656629.
Qi QR, Zhao XY, Zuo RJ, Wang TS, Gu XW, Liu JL, et al. Involvement of atypical transcription factor E2F8 in the polyploidization during mouse and human decidualization. Cell Cycle. 2015;14(12):1842–58. https://doi.org/10.1080/15384101.2015.1033593.
Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2(2):76–83. https://doi.org/10.1038/35000025.
Zhang XH, Liang X, Liang XH, Wang TS, Qi QR, Deng WB, et al. The mesenchymal-epithelial transition during in vitro decidualization. Reprod Sci. 2013;20(4):354–60. https://doi.org/10.1177/1933719112472738.
Ma XH, Hu SJ, Ni H, Zhao YC, Tian Z, Liu JL, et al. Serial analysis of gene expression in mouse uterus at the implantation site. J Biol Chem. 2006;281(14):9351–60. https://doi.org/10.1074/jbc.M511512200.
Ma WG, Song H, Das SK, Paria BC, Dey SK. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad Sci U S A. 2003;100(5):2963–8. https://doi.org/10.1073/pnas.0530162100.
Shah NM, Lai PF, Imami N, Johnson MR. Progesterone-related immune modulation of pregnancy and labor. Front Endocrinol (Lausanne). 2019;10:198. https://doi.org/10.3389/fendo.2019.00198.
Conneely OM, Mulac-Jericevic B, DeMayo F, Lydon JP, O’Malley BW. Reproductive functions of progesterone receptors. Recent Prog Horm Res. 2002;57:339–55. https://doi.org/10.1210/rp.57.1.339.
Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci U S A. 2003;100(17):9744–9. https://doi.org/10.1073/pnas.1732707100.
Arkaravichien W, Kendle KE. Critical progesterone requirement for maintenance of pregnancy in ovariectomized rats. J Reprod Fertil. 1990;90(1):63–70. https://doi.org/10.1530/jrf.0.0900063.
Csapo AI, Pulkkinen MO, Wiest WG. Effects of luteectomy and progesterone replacement therapy in early pregnant patients. Am J Obstet Gynecol. 1973;115(6):759–65. https://doi.org/10.1016/0002-9378(73)90517-6.
Ginsburg KA. Luteal phase defect. Etiology, diagnosis, and management. Endocrinol Metab Clin North Am. 1992;21(1):85–104.
Peyron R, Aubeny E, Targosz V, Silvestre L, Renault M, Elkik F, et al. Early termination of pregnancy with mifepristone (RU 486) and the orally active prostaglandin misoprostol. N Engl J Med. 1993;328(21):1509–13. https://doi.org/10.1056/NEJM199305273282101.
Funding
This work was supported by grants from the Yunnan Academic Leaders and Reserve Personnel (2017HB041), Key Projects of Basic and Applied Research in Yunnan Province (2018FA009), and the National Key Research and Development Program (2018YFC1002106).
Author information
Authors and Affiliations
Contributions
ZW planned and designed the research. XJT and HSZ finished the experiments. HXX and MW performed the data analysis. XJT and XMK wrote the manuscript. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The experiment was approved by the Ethics Committee of the First People’s Hospital of Yunnan Province. All patients provided written informed consent for their data to be used in the study. And, the study was carried out in accordance with the principles of the Declaration of Helsinki.
Competing Interests
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tang, X., Zheng, H., Xu, H. et al. NR4A1 Affects Endometrial Receptivity by Participating in Mesenchymal–Epithelial Transition of Endometrial Stromal Cells. Reprod. Sci. 29, 133–142 (2022). https://doi.org/10.1007/s43032-021-00792-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00792-z