Abstract
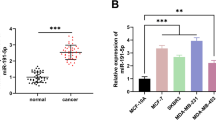
Tumor-associated macrophages (TAMs) are a major regulator in the development of endometrial cancer (EC). It was indicated that TAMs could crosstalk with cancer cells via transferring exosomes which carrying microRNAs (miRNAs). Firstly, we found that TAMs could promote the epithelial-mesenchymal transition (EMT) of EC cells and inhibit its apoptosis. Next, we further found that TAMs regulated the EMT and apoptosis of EC cells through transferring exosomes into EC cells. Then, lowly expressed miR-192-5p in TAMs-derived exosomes was proved. Moreover, our data demonstrated that upregulation of miR-192-5p in TAMs-derived exosomes could significantly promote the apoptosis of EC cells and impede its EMT. IRAK1 was proved to be a downstream target of miR-192-5p. Importantly, we indicated that miR-192-5p-overexpressed TAMs-derived exosomes regulated the EC cells apoptosis and EMT through inhibiting IRAK1/NF-κB signaling pathway. In addition, we also revealed that overexpression of miR-192-5p in TAMs-derived exosomes obviously limits the growth of tumors. Overall, in TAMs-derived exosomes, our data demonstrated that overexpression of miR-192-5p could effectively suppress the progression of EC. Our data provid a new target for EC treatment.







Similar content being viewed by others
Data Availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
McAlpine JN, Temkin SM, Mackay HJ. Endometrial cancer: not your grandmother's cancer. Cancer. 2016;122:2787–98.
Braun MM, Overbeek-Wager EA, Grumbo RJ. Diagnosis and management of endometrial cancer. Am Fam Physician. 2016;93:468–74.
Brooks RA, Fleming GF, Lastra RR, Lee NK, Moroney JW, Son CH, Tatebe K, Veneris JL. Current recommendations and recent progress in endometrial cancer. CA Cancer J Clin. 2019;69:258–79.
Kim J, Bae JS. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediators Inflamm. 2016;2016:6058147.
Sawa-Wejksza K, Kandefer-Szerszeń M. Tumor-associated macrophages as target for antitumor therapy. Arch Immunol Ther Exp (Warsz). 2018;66:97–111.
Chen Y, Song Y, Du W, Gong L, Chang H, Zou Z. Tumor-associated macrophages: an accomplice in solid tumor progression. J Biomed Sci. 2019;26:78.
Petty AJ, Yang Y. Tumor-associated macrophages: implications in cancer immunotherapy. Immunotherapy. 2017;9:289–302.
Gu S, Ni T, Wang J, Liu Y, Fan Q, Wang Y, Huang T, Chu Y, Sun X. CD47 blockade inhibits tumor progression through promoting phagocytosis of tumor cells by M2 polarized macrophages in endometrial cancer. J Immunol Res. 2018;2018:6156757.
Soeda S, Nakamura N, Ozeki T, Nishiyama H, Hojo H, Yamada H, Abe M, Sato A. Tumor-associated macrophages correlate with vascular space invasion and myometrial invasion in endometrial carcinoma. Gynecol Oncol. 2008;109:122–8.
Jing X, Peng J, Dou Y, Sun J, Ma C, Wang Q, Zhang L, Luo X, Kong B, Zhang Y, et al. Macrophage ERα promoted invasion of endometrial cancer cell by mTOR/KIF5B-mediated epithelial to mesenchymal transition. Immunol Cell Biol. 2019;97:563–76.
He C, Zheng S, Luo Y, Wang B. Exosome theranostics: biology and translational medicine. Theranostics. 2018;8:237–55.
Sasaki R, Kanda T. Exosomes and hepatocellular carcinoma: from bench to bedside. Int J Mol Sci. 2019;20(6):1406.
Lei B, He A, Chen Y, Cao X, Zhang P, Liu J, Ma X, Qian L, Zhang W. Long non-coding RNA RPPH1 promotes the proliferation, invasion and migration of human acute myeloid leukemia cells through down-regulating miR-330-5p expression. Excli j. 2019;18:824–37.
Li X, Tang M. Exosomes released from M2 macrophages transfer miR-221-3p contributed to EOC progression through targeting CDKN1B. Cancer Med. 2020;9:5976–88.
Cheng BY, Lau EY, Leung HW, Leung CO, Ho NP, Gurung S, Cheng LK, Lin CH, Lo RC, Ma S, et al. IRAK1 Augments cancer stemness and drug resistance via the AP-1/AKR1B10 signaling cascade in hepatocellular carcinoma. Cancer Res. 2018;78:2332–42.
Wee ZN, Yatim SM, Kohlbauer VK, Feng M, Goh JY, Bao Y, Lee PL, Zhang S, Wang PP, Lim E, et al. IRAK1 is a therapeutic target that drives breast cancer metastasis and resistance to paclitaxel. Nat Commun. 2015;6:8746.
Meng DF, Sun R, Liu GY, Peng LX, Zheng LS, Xie P, Lin ST, Mei Y, Qiang YY, Li CZ, et al. S100A14 suppresses metastasis of nasopharyngeal carcinoma by inhibition of NF-kB signaling through degradation of IRAK1. Oncogene. 2020;39:5307–22.
Genin M, Clement F, Fattaccioli A, Raes M, Michiels C. M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer. 2015;15:577.
Laviron M, Boissonnas A. Ontogeny of tumor-associated macrophages. Front Immunol. 2019;10:1799.
Yang Y, Ye YC. Crosstalk between hepatic tumor cells and macrophages via Wnt/β-catenin signaling promotes M2-like macrophage polarization and reinforces tumor malignant behaviors. Cell Death Dis. 2018;9:793.
Zhao S, Mi Y, Guan B, Zheng B, Wei P, Gu Y, Zhang Z, Cai S, Xu Y, Li X, et al. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J Hematol Oncol. 2020;13:156.
Komohara Y, Fujiwara Y, Ohnishi K, Takeya M. Tumor-associated macrophages: potential therapeutic targets for anti-cancer therapy. Adv Drug Deliv Rev. 2016;99:180–5.
Zhou J, Li X, Wu X, Zhang T, Zhu Q, Wang X, Wang H, Wang K, Lin Y, Wang X. Exosomes released from tumor-associated macrophages transfer miRNAs that induce a Treg/Th17 cell imbalance in epithelial ovarian cancer. Cancer Immunol Res. 2018;6:1578–92.
Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–79.
Zheng P, Chen L, Yuan X, Luo Q, Liu Y, Xie G, Ma Y, Shen L. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J Exp Clin Cancer Res. 2017;36:53.
Lan J, Sun L, Xu F, Liu L, Hu F, Song D, Hou Z, Wu W, Luo X, Wang J, et al. M2 macrophage-derived exosomes promote cell migration and invasion in colon cancer. Cancer Res. 2019;79:146–58.
Yang M, Qin X, Qin G, Zheng X. The role of IRAK1 in breast cancer patients treated with neoadjuvant chemotherapy. Onco Targets Ther. 2019;12:2171–80.
Rhyasen GW, Bolanos L, Fang J, Jerez A, Wunderlich M, Rigolino C, Mathews L, Ferrer M, Southall N, Guha R, et al. Targeting IRAK1 as a therapeutic approach for myelodysplastic syndrome. Cancer Cell. 2013;24:90–104.
Kong F, Liu Z. Inhibition of IRAK1 ubiquitination determines glucocorticoid sensitivity for TLR9-induced inflammation in macrophages. J Immunol. 2017;199:3654–67.
Ye ZH, Gao L, Wen DY, He Y, Pang YY, Chen G. Diagnostic and prognostic roles of IRAK1 in hepatocellular carcinoma tissues: an analysis of immunohistochemistry and RNA-sequencing data from the cancer genome atlas. Onco Targets Ther. 2017;10:1711–23.
Funding
This study was supported by the Inner Mongolia Autonomous Region People’s Hospital Fund (2019YN12) and Inner Mongolia Medical University million project joint project (YKD2018KJBW(LH)061).
Author information
Authors and Affiliations
Contributions
Conceptualization: Yilin Wang and Hongsheng Ma; methodology: Yilin Wang, Yajie Li and Hongsheng Ma; formal analysis and investigation: Yilin Wang and Rina Su; writing (original draft preparation): Yilin Wang; writing (review and editing): Hongsheng Ma, Yajie Li, and Rina Su; funding acquisition: Hongsheng Ma; resources: Hongsheng Ma; supervision: Yilin Wang, Hongsheng Ma, Yajie Li, and Rina Su.
Corresponding author
Ethics declarations
Ethics Approval
The approval of the animal experiment was granted by the Ethics Committee of Inner Mongolia People’s Hospital.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information

Supplementary Figure 1.
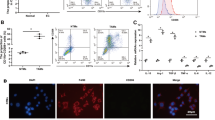
Detection of M2 macrophage-related cytokines. (A-C) The expression of Arg-1 mRNA, IL-10 mRNA and IL-4 mRNA in THP-1 cells (Ctrl group), M0 and TAMs was determined using qRT-PCR. *P < 0.05 compared with Ctrl group. (PNG 101 kb)

Supplementary Figure 2.
Effect of TAMs-derived exosomes on EC cells EMT. EC cells were treated with PBS, M0-conditional medium, and TAMs-conditional medium. Then, (A) western blot was executed to detect the expression of ZEB1 and N-cadherin. *P < 0.05 compared with Ctrl group. (B) Immunofluorescence was performed to detect the expression of E-cadherin and Vimentin in EC cells. (PNG 1460 kb)

Supplementary Figure 3.
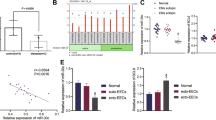
Detection of the cell proliferation of EC cells. (A) EC cells were treated with PBS, M0-conditional medium, and TAMs-conditional medium. Then, cell proliferation was determined using CCK-8 assay. *P < 0.05 compared with Ctrl group. (B) EC cells were treated with the TAMs-exo, negative mimic transfected TAMs-derived exosome, and miR-192-5p increased TAMs-derived exosomes. Then, CCK-8 was performed to detect the proliferation of EC cells. *P < 0.05 compared with Ctrl group. #P < 0.05 compared with TAMs-Exo group. (PNG 173 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Ma, H., Li, Y. et al. MiR-192-5p-Modified Tumor-Associated Macrophages-Derived Exosome Suppressed Endometrial Cancer Progression Through Targeting IRAK1/NF-κB Signaling. Reprod. Sci. 29, 436–447 (2022). https://doi.org/10.1007/s43032-021-00789-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00789-8