Abstract
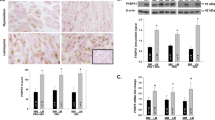
Uterine leiomyomas are benign, estrogen-sensitive, fibrotic smooth muscle cell tumors occurring in the uterine myometrium. Leiomyomas are a considerable health burden, with a lifetime prevalence of 80% and limited treatment options. Estrogen and progesterone have positive effects on leiomyoma growth, but little is known about the roles of other hormones. One hormone of interest is prolactin, as it has been described to be present and functional in leiomyomas. The current study investigates prolactin production within leiomyomas and its effects on myometrial cells. RNA isolation and quantitative-PCR of human leiomyoma samples relative to matched adjacent myometrium confirms significant expression of prolactin and dopamine receptor D2, a known regulator of prolactin production and release in the pituitary, with no difference in prolactin receptor expression. Immunohistochemistry confirms increased prolactin in leiomyomas compared to adjacent myometrium and uteri from women without leiomyomas. These results suggest that leiomyomas contain cells that produce prolactin, which may then promote signaling in leiomyoma cells to regulate leiomyoma development/growth. Accordingly, we find that prolactin robustly activates STAT5 and MAPK signaling in rat and human myometrial cell lines. Furthermore, prolactin stimulates expression of myofibroblast markers in rat myometrial cells. Our findings suggest that local prolactin production in leiomyomas may stimulate trans-differentiation of myometrial cells to myofibroblasts, which in turn contributes to the fibrotic nature of leiomyomas.







Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
Code Availability
Not applicable.
References
Stewart EA, Friedman AJ, Peck K, Nowak RA. Relative overexpression of collagen type I and collagen type III messenger ribonucleic acids by uterine leiomyomas during the proliferative phase of the menstrual cycle. J Clin Endocrinol Metab. 1994;79(3):900–6.
Cramer SF, Patel A. The frequency of uterine leiomyomas. Am J Clin Pathol. 1990;94(4):435–8.
Stewart EA. Uterine fibroids. Lancet. 2001;357(9252):293–8.
Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol. 2012;206(3):211-e1–9.
Ali M, Al-Hendy A. Selective progesterone receptor modulators for fertility preservation in women with symptomatic uterine fibroids. Biol Reprod. 2017;97(3):337–52.
Tasci Y, Caglar GS, Kayikcioglu F, Cengiz H, Yagci B, Gunes M. Treatment of menorrhagia with the levonorgestrel releasing intrauterine system: effects on ovarian function and uterus. Arch Gynecol Obstet. 2009;280(1):39–42.
Reis FM, Bloise E, Ortiga-Carvalho TM. Hormones and pathogenesis of uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2016;34:13–24.
Omar M, Laknaur A, Al-Hendy A, Yang Q. Myometrial progesterone hyper-responsiveness associated with increased risk of human uterine fibroids. BMC Womens Health. 2019;19(1):92.
Kim JJ, Sefton EC. The role of progesterone signaling in the pathogenesis of uterine leiomyoma. Mol Cell Endocrinol. 2012;358(2):223–31.
O’Connor KA, Ferrell R, Brindle E, Trumble B, Shofer J, Holman DJ, et al. Progesterone and ovulation across stages of the transition to menopause. Menopause. 2009;16(6):1178–87.
Lamminen S, Rantala I, Helin H, Rorarius M, Tuimala R. Proliferative activity of human uterine leiomyoma cells as measured by automatic image analysis. Gynecol Obstet Invest. 1992;34(2):111–4.
Sumitani H, Shozu M, Segawa T, Murakami K, Yang HJ, Shimada K, et al. In situ estrogen synthesized by aromatase P450 in uterine leiomyoma cells promotes cell growth probably via an autocrine/intracrine mechanism. Endocrinology. 2000;141(10):3852–61.
Bulun SE, Simpson ER, Word RA. Expression of the CYP19 gene and its product aromatase cytochrome P450 in human uterine leiomyoma tissues and cells in culture. J Clin Endocrinol Metab. 1994;78(3):736–43.
MacLeod RM. Influence of norepinephrine and catecholamine-depleting agents on the synthesis and release of prolactin and growth hormone. Endocrinology. 1969;85(5):916–23.
MacLeod RM, Fontham EH, Lehmeyer JE. Prolactin and growth hormone production as influenced by catecholamines and agents that affect brain catecholamines. Neuroendocrinology. 1970;6(5):283–94.
Chang A, Shin SH, Pang SC. Dopamine D2 receptor mediates both inhibitory and stimulatory actions on prolactin release. Endocrine. 1997;7(2):177–82.
Able AA, Burrell JA, Stephens JM. STAT5-Interacting Proteins: A Synopsis of Proteins that Regulate STAT5 Activity. Biology (Basel). 2017;6(1):20. https://doi.org/10.3390/biology6010020
Rani A, Murphy JJ. STAT5 in Cancer and Immunity. J Interferon Cytokine Res. 2016;36(4):226–37.
Van Etten RA. Aberrant cytokine signaling in leukemia. Oncogene. 2007;26(47):6738–49.
Daly DC, Walters CA, Prior JC, Kuslis ST, Chapitis J, Andreoli J, et al. Prolactin production from proliferative phase leiomyoma. Am J Obstet Gynecol. 1984;148(8):1059–63.
Nowak RA, Mora S, Diehl T, Rhoades AR, Stewart EA. Prolactin is an autocrine or paracrine growth factor for human myometrial and leiomyoma cells. Gynecol Obstet Invest. 1999;48(2):127–32.
Nowak RA, Rein MS, Heffner LJ, Friedman AJ, Tashjian AH Jr. Production of prolactin by smooth muscle cells cultured from human uterine fibroid tumors. J Clin Endocrinol Metab. 1993;76(5):1308–13.
Nohara A, Ohmichi M, Koike K, Jikihara H, Kimura A, Masuhara K, et al. Prolactin stimulates mitogen-activated protein kinase in human leiomyoma cells. Biochem Biophys Res Commun. 1997;238(2):473–7.
Mehine M, Kaasinen E, Heinonen HR, Makinen N, Kampjarvi K, Sarvilinna N, et al. Integrated data analysis reveals uterine leiomyoma subtypes with distinct driver pathways and biomarkers. Proc Natl Acad Sci USA. 2016;113(5):1315–20.
Herzog AG. Migraine with ectopic hyperprolactinemia from uterine fibroids. Neurology. 2000;55(1):148–9.
Sato H, Asami Y, Shiro R, Yasuda M, Imai S, Sakai R, et al. Resolution of dopamine agonist-resistant hyperprolactinemia by hysterectomy: a case report. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2018;34(3):199–201.
Sachdev S, Reyes MC, Snyder PJ. Ectopic Prolactin Secretion From a Uterine Leiomyoma. J Endocr Soc. 2020;4(4):bvaa035.
Cordiano V. Complete remission of hyperprolactinemia and erythrocytosis after hysterectomy for a uterine fibroid in a woman with a previous diagnosis of prolactin-secreting pituitary microadenoma. Ann Hematol. 2005;84(3):200–2.
Howe SR, Gottardis MM, Everitt JI, Goldsworthy TL, Wolf DC, Walker C. Rodent model of reproductive tract leiomyomata. Establishment and characterization of tumor-derived cell lines. Am J Pathol. 1995;146(6):1568–79.
Prizant H, Taya M, Lerman I, Light A, Sen A, Mitra S, et al. Estrogen maintains myometrial tumors in a lymphangioleiomyomatosis model. Endocr Relat Cancer. 2016;23(4):265–80.
Condon J, Yin S, Mayhew B, Word RA, Wright WE, Shay JW, et al. Telomerase immortalization of human myometrial cells. Biol Reprod. 2002;67(2):506–14.
Walker CL. Role of hormonal and reproductive factors in the etiology and treatment of uterine leiomyoma. Recent Prog Horm Res. 2002;57:277–94.
Bancroft FC, Levine L, Tashjian AH Jr. Control of growth hormone production by a clonal strain of rat pituitary cells. Stimulation by hydrocortisone J Cell Biol. 1969;43(3):432–41.
Stewart EA, Austin DJ, Jain P, Penglase MD, Nowak RA. RU486 suppresses prolactin production in explant cultures of leiomyoma and myometrium. Fertil Steril. 1996;65(6):1119–24.
Tworoger SS, Eliassen AH, Sluss P, Hankinson SE. A prospective study of plasma prolactin concentrations and risk of premenopausal and postmenopausal breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(12):1482–8.
Clevenger CV, Chang WP, Ngo W, Pasha TL, Montone KT, Tomaszewski JE. Expression of prolactin and prolactin receptor in human breast carcinoma. Evidence for an autocrine/paracrine loop. Am J Pathol. 1995;146(3):695–705.
Rose-Hellekant TA, Arendt LM, Schroeder MD, Gilchrist K, Sandgren EP, Schuler LA. Prolactin induces ERalpha-positive and ERalpha-negative mammary cancer in transgenic mice. Oncogene. 2003;22(30):4664–74.
Moore AB, Yu L, Swartz CD, Zheng X, Wang L, Castro L, et al. Human uterine leiomyoma-derived fibroblasts stimulate uterine leiomyoma cell proliferation and collagen type I production, and activate RTKs and TGF beta receptor signaling in coculture. Cell Commun Signal. 2010;8:10.
Richards RG, Brar AK, Frank GR, Hartman SM, Jikihara H. Fibroblast cells from term human decidua closely resemble endometrial stromal cells: induction of prolactin and insulin-like growth factor binding protein-1 expression. Biol Reprod. 1995;52(3):609–15.
Melli MS, Farzadi L, Madarek EO. Comparison of the effect of gonadotropin-releasing hormone analog (Diphereline) and Cabergoline (Dostinex) treatment on uterine myoma regression. Saudi Med J. 2007;28(3):445–50.
Vahdat M, Kashanian M, Ghaziani N, Sheikhansari N. Evaluation of the effects of cabergoline (Dostinex) on women with symptomatic myomatous uterus: a randomized trial. Eur J Obstet Gynecol Reprod Biol. 2016;206:74–8.
Agarwal N, Machiels JP, Suarez C, Lewis N, Higgins M, Wisinski K, et al. Phase I Study of the Prolactin Receptor Antagonist LFA102 in Metastatic Breast and Castration-Resistant Prostate Cancer. Oncologist. 2016;21(5):535–6.
Funding
NIH grant R01CA193583 (S.R.H.) funded the majority of this work. B.M. was supported by T32AI007285. B.B. was supported by the Richard W. and Mae Stone Goode Grant Program.
Author information
Authors and Affiliations
Contributions
A.D. performed the majority of the studies in this manuscript, wrote the initial draft of the manuscript, and created most of the figures. C.S., B.M. A.A., and I.O. also performed experiments presented in the manuscript. A.R.K. performed the prolactin-induced fibroblast factor studies. M.T. served as an advisor for these studies. B.B. came up with the concept and with help of D.K. provided the uterine samples. S.R.H. supervised the project and assisted with data interpretation and presentation, as well as writing of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest/Competing Interests
The authors declare that they have no competing interests.
Ethics Approval and Consent to Participate
All patients were provided informed consent. IRB and IACUC approval was obtained for this study. All samples were de-identified.
Consent for Publication
All work was performed in the Hammes laboratory at the University of Rochester Medical Center. All authors consent for publication.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
DiMauro, A., Seger, C., Minor, B. et al. Prolactin is Expressed in Uterine Leiomyomas and Promotes Signaling and Fibrosis in Myometrial Cells. Reprod. Sci. 29, 2525–2535 (2022). https://doi.org/10.1007/s43032-021-00741-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00741-w