Abstract
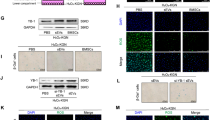
This study aimed to evaluate the effects of growth and differentiation factor-9 (GDF-9) on the morphology, activation, apoptosis, and granulosa cell proliferation of ovine preantral follicles cultured within ovarian tissue slices and to verify whether GDF-9 could influence follicular activation through the phosphatidylinositol 3-kinase/protein kinase B/forkhead box O3a (PI3K/Akt/FOXO3a) pathway. Ovine ovarian fragments were cultured in α-MEM+ or α-MEM+ with GDF-9 (1, 50, 100, 200, or 400 ng/ml) for 7 days. Apoptosis and cell proliferation were analyzed. Next, the activation of the PI3K was inhibited with LY294002, and immunostaining for p-Akt and p-FOXO3a proteins was assessed. The concentration of 50 ng/ml GDF-9 had (P < 0.05) more morphologically normal follicles compared to all treatments, except 1 ng/ml GDF-9. Moreover, 50 ng/ml GDF-9 increased primordial follicle activation compared to all treatments, except α-MEM+ and 1 ng/ml GDF-9. However, the concentration of 50 ng/ml GDF-9 showed higher cell proliferation and lower apoptosis than α-MEM+ and 1 ng/ml GDF-9 treatments. Culture of the ovarian tissue with LY294002 inhibited the activation of primordial follicles and reduced p-Akt immunostaining in both α-MEM+ and 50 ng/ml GDF-9 treatments. In addition, after culture with LY294002, the percentage of oocytes with nuclear p-FOXO3 was higher in 50 ng/ml GDF-9 than in the control medium (α-MEM+). In conclusion, after culture of ovine ovarian cortical slices, the addition of 50 ng/ml GDF-9 reduces follicular apoptosis and promotes granulosa cell proliferation likely through the involvement of phosphorylated Akt and FOXO3a.







Similar content being viewed by others
References
Shea LD, Woodruff TK, Shikanov A. Bioengineering the ovarian follicle microenvironment. Annu Rev Biomed Eng. 2014;16:29–52.
Monniaux D, Clément F, Dalbiès-Tran R, et al. The ovarian reserve of primordial follicles and the dynamic reserve of antral growing follicles: what is the link? Biol Reprod. 2014;90:85.
Li J, Kawamura K, Cheng Y, Liu S, Klein C, Liu S, et al. Activation of dormant ovarian follicles to generate mature eggs. Proc Natl Acad Sci U S A. 2010;107:10280–4.
Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci U S A. 2013;110:17474–9.
Vitt UA, Hayashi M, Klein C, Hsueh AJ. Growth differentiation factor-9 stimulates proliferation but suppresses the follicle-stimulating hormone-induced differentiation of cultured granulosa cells from small antral and preovulatory rat follicles. Biol Reprod. 2000;62:370–7.
Orisaka M, Orisaka S, Jiang JY, Craig J, Wang Y, Kotsuji F, et al. Growth differentiation factor 9 is antiapoptotic during follicular development from preantral to early antral stage. Mol Endocrinol. 2006;20:2456–68.
Dipaz-Berrocal DJ, Sá NAR, Guerreiro DD, Celestino JJH, Leiva-Revilla J, Alves BG, et al. Refining insulin concentrations in culture medium containing growth factors BMP15 and GDF9: an in vitro study of the effects on follicle development of goats. Anim Reprod Sci. 2017;185:118–27.
Marino PC, Bizarro-Silva C, Búfalo I, Rosa CO, Gonçalves GR, Lisboa LA, et al. Growth and differentiation factor-9 supplementation affects viability and morphology of preantral follicles in equine ovarian fragments during short-term in vitro culture. Braz Arch Biol Technol. 2019;62:e19180346.
Hreinsson JG, Scott JE, Rasmussen C, Swahn ML, Hsueh ALW, Hovatta O. Growth differentiation factor-9 promotes the growth, development, and survival of human ovarian follicles in organ culture. J Clin Endocrinol Metab. 2002;87:316–21.
Martins FS, Celestino JJH, Saraiva MVA, Matos MHT, Bruno JB, Rocha-Junior CMC, et al. Growth and differentiation factor-9 stimulates activation of goat primordial follicles in vitro and their progression to secondary follicles. Reprod Fertil Dev. 2008;20:916–24.
Tang K, Yang W, Li X, Wu C, Sang L, Yang L. GDF-9 and bFGF enhance the effect of FSH on the survival, activation, and growth of cattle primordial follicles. Anim Reprod Sci. 2012;131:129–34.
Almeida AP, Saraiva MVA, Araújo VR, Magalhães DM, Duarte ABG, Frota IMA, et al. Expression of growth and differentiation factor 9 (GDF-9) and its effect on the in vitro culture of caprine preantral ovarian follicles. Small Rumin Res. 2011;100:169–76.
Cook-Andersen H, Curnow KJ, Su HI, Chang RJ, Shimasaki S. Growth and differentiation factor 9 promotes oocyte growth at the primary but not the early secondary stage in three-dimensional follicle culture. J Assist Reprod Genet. 2016;33:1067–77.
Monte APO, Santos JM, Menezes VG, Gouveia BB, Lins LBG, Barberino RS, et al. Growth differentiation factor-9 improves development, mitochondrial activity and meiotic resumption of sheep oocytes after in vitro culture of secondary follicles. Reprod Domest Anim. 2019;54:1169–76.
Bodensteiner KJ, Clay CM, Moeller CL, Sawyer HR. Molecular cloning of the ovine growth/differentiation factor-9 gene and expression of growth/differentiation factor-9 in ovine and bovine ovaries. Biol Reprod. 1999;60:381–6.
Mery L, Lefevre A, Benchaib M, Demirci B, Salle B, Guerin JF, et al. Follicular growth in vitro: detection of growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15) during in vitro culture of ovine cortical slices. Mol Reprod Dev. 2007;74:767–74.
Scaramuzzi RJ, Baird BM, Campbell BK, et al. Regulation of folliculogenesis and the determination of ovulation rate in ruminants. Reprod Fertil Dev. 2011;23:444–67.
Kona SSR, Praveen Chakravarthi V, Siva Kumar AVN, Srividya D, Padmaja K, Rao VH. Quantitative expression patterns of GDF9 and BMP15 genes in sheep ovarian follicles grown in vivo or cultured in vitro. Theriogenology. 2015;85:315–22.
Dong J, Albertine DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–5.
Mazerbourg S, Klein C, Roh J, Kaivo-Oja N, Mottershead DG, Korchynskyi O, et al. Growth differentiation factor-9 signaling is mediated by the type I receptor, activin receptor-like kinase-5. Mol Endocrinol. 2004;18:653–65.
Huang Q, Cheung AP, Zhang Y, Huang HF, Auersperg N, Leung PCK. Effects of growth differentiation factor 9 on cell cycle regulators and ERK42/44 in human granulosa cell proliferation. Am J Physiol Endocrinol Metab. 2009;296:1344–53.
Reader KL, Heath DA, Lun S, McIntosh CJ, Western AH, Roger RPL, et al. Signalling pathways involved in the cooperative effects of ovine and murine GDF9 CBMP15-stimulated thymidine uptake by rat granulosa cells. Reproduction. 2011;142:123–31.
Bertoldo MJ, Walters KA, Ledger WL, Gilchrist RB, Mermillod P, Locatelli Y. In-vitro regulation of primordial follicle activation: challenges for fertility preservation strategies. Reprod Biomed Online. 2018;36:491–9.
Nagamatsu G, Shimamoto S, Hamazaki N, Nishimura Y, Hayashi K. Mechanical stress accompanied with nuclear rotation is involved in the dormant state of mouse oocytes. Sci Adv. 2019;5:9960.
Liu Z, Castrillon DH, Zhou W, Richards JS. FOXO1/3 depletion in granulosa cells alters follicle growth, death and regulation of pituitary FSH. Mol Endocrinol. 2013;27:238–52.
Matsuda F, Inoue N, Maeda A, Cheng Y, Sai T, Gonda H, et al. Expression and function of apoptosis initiator FOXO3 in granulosa cells during follicular atresia in pig ovaries. J Reprod Dev. 2011;57:151–8.
Soares MAA, Costa JJN, Vasconcelos GL, et al. Effects of frutalin on early follicle morphology, ultrastructure and gene expression in cultured goat ovarian cortical tissue. Histol Histopathol. 2018;33:41–53.
Bezerra MÉS, Barberino RS, Menezes VG, Gouveia BB, Macedo TJS, Santos JMS, et al. Insulin-like growth factor-1 (IGF-1) promotes primordial follicle growth and reduces DNA fragmentation through the phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) signalling pathway. Reprod Fertil Dev. 2018;30:1503–14.
Silva JR, Van Den Hurk R, Matos MHT, et al. Influences of FSH and EGF on primordial follicles during in vitro culture of caprine ovarian cortical tissue. Theriogenology. 2004;61:1691–704.
Langbeen A, Ginneken CV, Fransen E, Bosmans E, Leroy JLMR, Bols PEJ. Morphometrical analysis of preantral follicular survival of VEGF-treated bovine ovarian cortex tissue following xenotransplantation in an immune deficient mouse model. Anim Reprod Sci. 2016;168:73–85.
Cecconi S, Mauro A, Cellini V, Patacchiola F. The role of Akt signalling in the mammalian ovary. Int J Dev Biol. 2012;56:809–17.
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68.
Brunet A, Kanai F, Stehn J, Xu J, Sarbassova D, Frangioni JV, et al. 14-3-3 transits to the nucleus and participates in dynamic nucleocytoplasmic transport. J Cell Biol. 2002;156:817–28.
Bertoldo MJ, Bernard J, Duffard N, Tsikis G, Alves S, Calais L, et al. Inhibitors of c-Jun phosphorylation impede ovine primordial follicle activation. Mol Hum Reprod. 2016;22:338–49.
Kim S, Ebbert K, Cordeiro MH, Romero M, Zhu J, Serna VA, et al. Cell autonomous phosphoinositide 3-kinase activation in oocytes disrupts normal ovarian function through promoting survival and overgrowth of ovarian follicles. Endocrinology. 2015;156:1464–76.
Picton HM. Activation of follicle development: the primordial follicle. Theriogenology. 2001;55:1193–210.
Braw-Tal R. The initiation of follicle growth: the oocyte or the somatic cells? Mol Cell Endocrinol. 2002;187:11–8.
Zhang H, Risal S, Gorre N, Busayavalasa K, Li X, Shen Y, et al. Somatic cells initiate primordial follicle activation and govern the development of dormant oocytes in mice. Curr Biol. 2014;24:2501–8.
Braw-Tal R, Yossefi S. Studies in vivo and in vitro on the initiation of follicle growth in the bovine ovary. J Reprod Fertil. 1997;109:165–71.
Spicer LJ, Aad PY, Allen D, Mazerbourg S, Hsueh AJ. Growth differentiation factor-9 has divergent effects on proliferation and steroidogenesis of bovine granulosa cells. J Endocrinol. 2006;189:329–39.
Vitt UA, McGee EA, Hayashi M, Hsueh AJ. In vivo treatment with GDF-9 stimulates primordial and primary follicle progression and theca cell marker CYP17 in ovaries of immature rats. Endocrinology. 2000;141(10):3814–20.
Workman P, Clarke PA, Raynaud FI, Montfort RLMV. Drugging the PI3 Kinome: from chemical tools to drugs in the clinic. Cancer Res. 2010;15:2146–57.
Lan ZJ, Krause MS, Redding SD, Li X, Wu GZ, Zhou HX, et al. Selective deletion of Pten in theca-interstitial cells leads to androgen excess and ovarian dysfunction in mice. Mol Cell Endocrinol. 2017;444:26–37.
Zhao Q, Ma Y, Sun NX, Ye C, Zhang Q, Sun SH, et al. Exposure to bisphenol a at physiological concentrations observed in Chinese children promotes primordial follicle growth through the PI3K/Akt pathway in an ovarian culture system. Toxicol in Vitro. 2014;28:1424–9.
Yin N, Wang Y, Lu X, Liu R, Zhang W, et al. HPMSC transplantation restoring ovarian function in premature ovarian failure mice is associated with change of Th17/Tc17 and Th17/Treg cell ratios through the PI3K/Akt signal pathway. Stem Cell Res Ther. 2018;9:1–14.
Yan W, Zhou S, Shen W, Cheng J, Yuan S, Ye S, et al. Suppression of SEMA6C promotes pre antral follicles atresia with decreased cell junctions in mice ovaries. J Cell Physiol. 2019;234:4934–43.
Gharbi SI, Zvelebil MJ, Shuttleworth SJ, Hancox T, Saghir N, Timms JF, et al. Exploring the specificity of the PI3K family inhibitor LY294002. Biochem J. 2007;404:15–21.
Touil Y, Zuliani T, Wolowczuk I, Kuranda K, Prochazkova J, Andrieux J, et al. The PI3K/AKT signaling pathway controls the quiescence of the low-Rhodamine123-retention cell compartment enriched for melanoma stem cell activity. Stem Cells. 2013;31:641–51.
Zhao HF, Wang J, To SST. The phosphatidylinositol 3-kinase/Akt and c-Jun N-terminal kinase signaling in cancer: Alliance or contradiction? Int J Oncol. 2015;47:429–36.
Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19:142–9.
Sunters A, Madureira PA, Pomeranz KM, Aubert M, Brosens JJ, Cook SJ, et al. Paclitaxel-induced nuclear translocation of FOXO3a in breast cancer cells is mediated by c-Jun NH2-terminal kinase and Akt. Cancer Res. 2006;66:212–21.
Sunayama J, Tsuruta F, Masuyama N, Gotoh Y. JNK antagonizes Akt-mediated survival signals by phosphorylating 14-3-3. J Cell Biol. 2005;170:295–304.
Zhao Z, Wang J, Tang J, Liu X, Zhong Q, Wang F, et al. JNK- and Akt-mediated Puma expression in the apoptosis of cisplatin-resistant ovarian cancer cells. Biochem J. 2012;444:291–301.
Acknowledgments
M.H.T. Matos is supported by a grant from CNPq (310712/2015-7).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Monte, A.P.O., Bezerra, M.É.S., Menezes, V.G. et al. Involvement of Phosphorylated Akt and FOXO3a in the Effects of Growth and Differentiation Factor-9 (GDF-9) on Inhibition of Follicular Apoptosis and Induction of Granulosa Cell Proliferation After In Vitro Culture of Sheep Ovarian Tissue. Reprod. Sci. 28, 2174–2185 (2021). https://doi.org/10.1007/s43032-020-00409-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-020-00409-x