Abstract
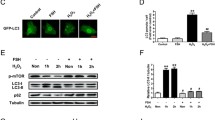
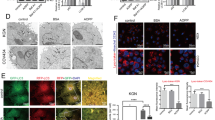
Oxidative stress-induced granulosa cell (GC) death is a major cause of follicular atresia. As the major types of programmed cell death, autophagy and apoptosis have been observed in response to H2O2-mediated oxidative stress and have been demonstrated to be responsible for porcine GC death. To date, however, the cellular reactions linking autophagy to the apoptosis of porcine GC under oxidative stress are still poorly understood. Porcine GC were treated with H2O2, and autophagic flux was examined by western blotting. Cell viability and cell death assays were performed after cotreatment of porcine GC with autophagy activator (rapamycin) or inhibitor (3-methyladenine, 3-MA) together with H2O2. We revealed that short exposure (1–3 h) of porcine GC to H2O2 dramatically increased autophagic flux (1.8- to 2.5-fold over that in the control), whereas 6–12 h prolonged treatment decreased autophagy but elevated the caspase-3 activity and GC apoptotic rate. Furthermore, we showed that pretreatment with rapamycin exacerbated H2O2-mediated cytotoxicity and caspase-3 activation but that 3-MA or siRNAs specific for Beclin 1 and Atg7 genes ameliorated H2O2-mediated GC apoptosis. Together, our results indicate that autophagy plays a pivotal role in H2O2-mediated porcine GC apoptosis. Importantly, we show that the early induction of autophagic flux contributes to oxidative stress-induced apoptosis in porcine GC. The results also suggest that regulating the autophagy response in porcine GC under oxidative stress might be a new strategy for abnormal follicular atresia.








Similar content being viewed by others
References
Agarwal A, Sharma R, Gupta S, Harlev A, Ahmad G, Plessis SSD, Esteves SC, Wang SM, Durairajanayagam D. Oxidative stress in human reproduction. 2017.
Ashok A, Anamar AM, Premkumar BJ, Amani S, Sajal G. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol Rb E. 2012;10:1–31.
Budovskaya YV, Stephan JS, Reggiori F, Klionsky DJ, Herman PK. The Ras/cAMP-dependent protein kinase signaling pathway regulates an early step of the autophagy process in Saccharomyces cerevisiae. J Biol Chem. 2004;279:20663–71.
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett JF, Kubista M, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–22.
Castino R, Bellio N, Follo C, Murphy D, Isidoro C. 3 Inhibition of PI3k class III-dependent autophagy prevents apoptosis and necrosis by oxidative. Toxicol Sci. 2010;117:152–62.
Chan KA, Jazwiec PA, Gohir W, Petrik JJ, Sloboda DM. Maternal nutrient restriction impairs young adult offspring ovarian signaling resulting in reproductive dysfunction and follicle loss. Biol Reprod. 2018;98.
Chen Y, Mcmillan-Ward E, Kong J, Israels SJ, Gibson SB. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ. 2008;15:171–82.
Chen X, Xu J, Liu D, Sun Y, Qian G, Xu S, et al. The aggravating effect of selenium deficiency on T-2 toxin-induced damage on primary cardiomyocyte results from a reduction of protective autophagy. Chem Biol Interact.
Choi J, Jo M, Lee E, Choi D. Induction of apoptotic cell death via accumulation of autophagosomes in rat granulosa cells. Fertil Steril. 2011;95:1482–6.
Choi J, Jo M, Lee E, Choi D. AKT is involved in granulosa cell autophagy regulation via mTOR signaling during rat follicular development and atresia. Reproduction. 2014;147:73–80.
Chu YL, Xu YR, Yang WX, Sun Y. The role of FSH and TGF-beta superfamily in follicle atresia. Aging (Albany NY). 2018;10:305–21.
Egger L, Schneider J, Rhême C, Tapernoux M, Häcki J, Borner C. Serine proteases mediate apoptosis-like cell death and phagocytosis under caspase-inhibiting conditions. Cell Death Differ. 2003;10:1188–203.
Fleming A, Noda T, Yoshimori T, Rubinsztein DC. Chemical modulators of autophagy as biological probes and potential therapeutics. Nat Chem Biol. 2011;7:9–17.
Hale BJ, Hager CL, Seibert JT, Selsby JT, Baumgard LH, Keating AF, et al. Heat stress induces autophagy in pig ovaries during follicular development. Biol Reprod. 2017;97:426–37.
Hoang YD, Nakamura BN, Ulrike L. Follicle-stimulating hormone and estradiol interact to stimulate glutathione synthesis in rat ovarian follicles and granulosa cells. Biol Reprod. 2009;81:636–46.
Huang Q, Ou YS, Tao Y, Yin H, Tu PH. Apoptosis and autophagy induced by pyropheophorbide-α methyl ester-mediated photodynamic therapy in human osteosarcoma MG-63 cells. Apoptosis. 2016;21:749–60.
Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–8.
Kim YC, Guan KL. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest. 2015;125:25–32.
Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–41.
Li X, Li X, Wang J, Ye Z, Li J. Oridonin up-regulates expression of P21 and induces autophagy and apoptosis in human prostate cancer cells. Int J Biol Ences. 2012;8:901–12.
Li L, Tan J, Miao Y, Lei P, Zhang Q. ROS and autophagy: interactions and molecular regulatory mechanisms. Cell Mol Neurobiol. 2015;35:615–21.
Lin F, Li R, Pan ZX, Zhou B, Yu DB, Wang XG, et al. miR-26b promotes granulosa cell apoptosis by targeting ATM during follicular atresia in porcine ovary. Plos One. 2012;7:e38640.
Lucile E, Mélanie D, Marina G, Véronique RH, Bernard G, Mihayl V, et al. Autophagy is involved in T cell death after binding of HIV-1 envelope proteins to CXCR4. J Clin Investig. 2006;116:2161.
Manabe N (2003) Ovarian follicle selection in mammalian ovaries: regulatory mechanisms of granulosa cell apoptosis during follicular atresia. Ovary.
Manabe N, Goto Y, Matsudaminehata F, Inoue N, Maeda A, Sakamaki K, et al. Regulation mechanism of selective atresia in porcine follicles: regulation of granulosa cell apoptosis during atresia. J Reprod Dev. 2004;50:493–514.
Manoj K, Dhananjay P, Alka K, Ammini AC, Pankaj T, Rima D. Nucleotide variations in mitochondrial DNA and supra-physiological ROS levels in cytogenetically normal cases of premature ovarian insufficiency. Arch Gynecol Obstet. 2010;282:695–705.
Matsuda F, Inoue N, Manabe N, Ohkura S. Follicular growth and atresia in mammalian ovaries: regulation by survival and death of granulosa cells. J Reprod Dev. 2012;58:44–50.
Ming-Feng W, Min-Wei C, Ke-Cheng C, Pei-Jen L, Susan Yun-Fan L, Shih-Chieh H, et al. Autophagy promotes resistance to photodynamic therapy-induced apoptosis selectively in colorectal cancer stem-like cells. Autophagy. 2014;10:1179–92.
Miyun TT, Ulrike L. Opposing effects of glutathione depletion and follicle-stimulating hormone on reactive oxygen species and apoptosis in cultured preovulatory rat follicles. Endocrinology. 2006;147:1224–36.
Qinwen W, Zhengtang C, Xinwei D, Shengbing H. Induction of autophagy-dependent apoptosis by the survivin suppressant YM155 in prostate cancer cells. Cancer Lett. 2011;302:29–36.
Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24:R453–62.
Shen M, Jiang Y, Guan Z, Cao Y, Li L, Liu H, et al. Protective mechanism of FSH against oxidative damage in mouse ovarian granulosa cells by repressing autophagy. Autophagy. 2017:00–0.
Shen M, Lin F, Zhang J, Tang Y, Chen WK, Liu H. Involvement of the up-regulated FoxO1 expression in follicular granulosa cell apoptosis induced by oxidative stress. J Biol Chem. 2012;287:25727–40.
Shinji S, Yukiko S, Yoko I, Sumihiro K, Takahiro F, Isei T, et al. Caffeine induces apoptosis by enhancement of autophagy via PI3K/Akt/mTOR/p70S6K inhibition. Autophagy. 2011;7:176–87.
Tekirdag KA, Korkmaz G, Ozturk DG, Agami R, Gozuacik D. MIR181A regulates starvation- and rapamycin-induced autophagy through targeting of ATG5. Autophagy. 2013;9:374–85.
Thoreen, CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. 2009;284:8023–8032.
Wang Q, Chen Z, Diao X, Huang S. Induction of autophagy-dependent apoptosis by the survivin suppressant YM155 in prostate cancer cells. Cancer Lett. 2011;302:29–36.
Wang D, Wang Y. Gasdermin D-mediated cell pyroptosis presents in the ovary of hyperandrogen-induced PCOS rats. Fertil Steril.
Wang Y, Zhu WG, Zhao Y. Autophagy substrate SQSTM1/p62 regulates chromatin ubiquitination during the DNA damage response. Autophagy. 2017;13:212–3.
Wei MF, Chen MW, Chen KC, Lou PJ, Lin YF, Hung SC, et al. Autophagy promotes resistance to photodynamic therapy-induced apoptosis selectively in colorectal cancer stem-like cells. Autophagy. 2014;10:1179–92.
Wei Q, Shi F. Cleavage of poly (ADP-ribose) polymerase-1 is involved in the process of porcine ovarian follicular atresia. Anim Reprod Sci. 2013;138:282–91.
Wu YT, Tan HL, Shui G, Bauvy C, Huang Q, Wenk MR, et al. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J Biol Chem. 2010;285:10850–61.
Yan Z, Dai Y, Fu H, Zheng Y, Bao D, Yin Y, et al. Curcumin exerts a protective effect against premature ovarian failure in mice. J Mol Endocrinol. 2018;60:JME-17-0214.
Yang Y, Cheung HH, Zhang C, Wu J, Chan WY. Melatonin as potential targets for delaying ovarian aging. Curr Drug Targets. 2019;20:16–28.
Yu J, Yaba A, Kasiman C, Thomson T, Johnson J. mTOR controls ovarian follicle growth by regulating granulosa cell proliferation. PLoS One. 2011;6:e21415.
Yuan K, Huang C, Fox J, Laturnus D, Carlson E, Zhang B, et al. Autophagy plays an essential role in the clearance of Pseudomonas aeruginosa by alveolar macrophages. J Cell Sci. 2012;125:507–15.
Zhang JQ, Gao BW, Guo HX, Ren QL, Wang XW, Chen JF, et al. miR-181a promotes porcine granulosa cell apoptosis by targeting TGFBR1 via the activin signaling pathway. Mol Cell Endocrinol. 2020;499:110603.
Zhang JQ, Gao BW, Wang J, Ren QL, Chen JF, Ma Q, et al. Critical role of FoxO1 in granulosa cell apoptosis caused by oxidative stress and protective effects of grape seed procyanidin B2. Oxidative Med Cell Longev. 2016;2016:1–16.
Zhang H, Kong XJ. Oxidative stress induces parallel autophagy and mitochondria dysfunction in human glioma U251 cells. Toxicol Sci. 2009;110:376–88.
Zhang JQ, Shen M, Zhu CC, Yu FX, Liu ZQ, Ally N, et al. 3-Nitropropionic acid induces ovarian oxidative stress and impairs follicle in mouse. PLoS One. 2014;9:e86589.
Zhang J, Xu Y, Liu H, Pan Z. MicroRNAs in ovarian follicular atresia and granulosa cell apoptosis. Reprod Biol Endocrinol. 2019;17:9.
Acknowledgments
The authors would like to thank 2 anonymous reviewers for their constructive comments and suggestions on an earlier version of the manuscript.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this study was supported by the Science and Technology Innovation and Creativity Project of Henan Academy of Agricultural Sciences (NO.2020CX06) and the Independent Innovation Fund Project in Henan Academy of Agricultural Science (NO.2020ZC36).
Author information
Authors and Affiliations
Contributions
Jia-Qing Zhang and Bao-Song Xing conceived and designed the experiments. Qiao-Ling Ren, Jun-Feng Chen, and Bin-Wen Gao performed the experiments. Xian-Wei Wang and Zi-Jing Zhang analyzed the data. Ze-Jun Xu assisted us to collect samples and supplement the tests. Jia-Qing Zhang and Bao-Song Xing wrote the manuscript.
Corresponding author
Ethics declarations
Animal procedures were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee of Animal Husbandry and Veterinary Science, Henan Academy of Agricultural Sciences. During the procedure, electrodes were used to ensure that the pigs were in an unconscious state, and then the ovaries were removed.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 170 kb)
Rights and permissions
About this article
Cite this article
Zhang, JQ., Ren, QL., Chen, JF. et al. Autophagy Contributes to Oxidative Stress-Induced Apoptosis in Porcine Granulosa Cells. Reprod. Sci. 28, 2147–2160 (2021). https://doi.org/10.1007/s43032-020-00340-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-020-00340-1