Abstract
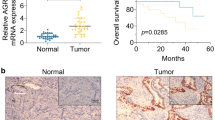
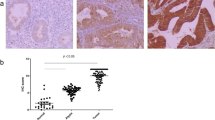
Claudin-2 (CLDN-2) is a leaky-type tight junction protein, and its overexpression increases tumorigenesis of some types of cancer cells. In the present study, to examine the possibility of targeting CLDN-2 in the therapy for endometrioid endometrial adenocarcinoma, we investigated the regulation and role of CLDN-2 in endometriosis and endometrioid endometrial adenocarcinoma. In endometrioid endometrial adenocarcinoma tissues, marked upregulation of CLDN-2 was observed together with malignancy, while in endometriosis tissues, a change in the localization of CLDN-2 was observed. In cells of the endometrial adenocarcinoma cell line Sawano, which highly express CLDN-2, downregulation of CLDN-2 induced by the siRNA upregulated the epithelial barrier and inhibited cell migration. Furthermore, the downregulation of CLDN-2 affected the cell cycle and inhibited cell proliferation. In Sawano cells cultured with high-glucose medium, CLDN-2 expression was downregulated at the mRNA and protein levels. The high-glucose medium upregulated the epithelial barrier, cell proliferation, and migration, and inhibited cell invasion. The histone deacetylase (HDAC) inhibitor tricostatin A (TSA), which has antitumor effects, downregulated CLDN-2 expression, cell proliferation, invasion, and migration, and upregulated the epithelial barrier. The mitochondrial respiration level, an indicator of cancer metabolism, was downregulated by CLDN-2 knockdown and upregulated by the high-glucose condition. Taken together, these results indicated that overexpression of CLDN-2 closely contributed to the malignancy of endometrioid endometrial adenocarcinoma. Downregulation of CLDN-2 via the changes of the glucose concentration and treatment with HDAC inhibitors may be important in the therapy for endometrial cancer.






Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86.
Lortet-Tieulent J, Ferlay J, Bray F, Jemal A. International patterns and trends in endometrial cancer incidence, 1978-2013. J Natl Cancer Inst. 2018;110(4):354–61.
Angelow S, Ahlstrom R, Yu AS. Biology of claudins. Am J Physiol Renal Physiol. 2008;295(4):F867–76.
Furuse M, Tsukita S. Claudins in occluding junctions of humans and flies. Trends Cell Biol. 2006;16(4):181–8.
Matter K, Balda MS. Signalling to and from tight junctions. Nat Rev Mol Cell Biol. 2003;4(3):225–36.
Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141(7):1539–50.
Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2(4):285–93.
Mineta K, Yamamoto Y, Yamazaki Y, Tanaka H, Tada Y, Saito K, et al. Predicted expansion of the claudin multigene family. FEBS Lett. 2011;585(4):606–12.
Turksen K, Troy TC. Barriers built on claudins. J Cell Sci. 2004;117(Pt 12):2435–47.
Leech AO, Cruz RG, Hill AD, Hopkins AM. Paradigms lost-an emerging role for over-expression of tight junction adhesion proteins in cancer pathogenesis. Ann Transl Med. 2015;3(13):184.
Ikari A, Sato T, Watanabe R, Yamazaki Y, Sugatani J. Increase in claudin-2 expression by an EGFR/MEK/ERK/c-Fos pathway in lung adenocarcinoma A549 cells. Biochim Biophys Acta. 2012;1823(6):1110–8.
Halász J, Holczbauer A, Páska C, Kovács M, Benyó G, Verebély T, et al. Claudin-1 and claudin-2 differentiate fetal and embryonal components in human hepatoblastoma. Hum Pathol. 2006;37(5):555–61.
Kinugasa T, Huo Q, Higashi D, Shibaguchi H, Kuroki M, Tanaka T, et al. Selective up-regulation of claudin-1 and claudin-2 in colorectal cancer. Anticancer Res. 2007;27(6A):3729–34.
Xin S, Huixin C, Benchang S, Aiping B, Jinhui W, Xiaoyan L, et al. Expression of Cdx2 and claudin-2 in the multistage tissue of gastric carcinogenesis. Oncology. 2007;73(5–6):357–65.
Ikari A, Watanabe R, Sato T, Taga S, Shimobaba S, Yamaguchi M, et al. Nuclear distribution of claudin-2 increases cell proliferation in human lung adenocarcinoma cells. Biochim Biophys Acta. 2014;1843(9):2079–88.
Ikari A, Sato T, Takiguchi A, Atomi K, Yamazaki Y, Sugatani J. Claudin-2 knockdown decreases matrix metalloproteinase-9 activity and cell migration via suppression of nuclear Sp1 in A549 cells. Life Sci. 2011;88(13–14):628–33.
Tabariès S, Dong Z, Annis MG, Omeroglu A, Pepin F, Ouellet V, et al. Claudin-2 is selectively enriched in and promotes the formation of breast cancer liver metastases through engagement of integrin complexes. Oncogene. 2011;30(11):1318–28.
Friberg E, Orsini N, Mantzoros CS, Wolk A. Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia. 2007;50(7):1365–74.
Lacey JV Jr, Chia VM. Endometrial hyperplasia and the risk of progression to carcinoma. Maturitas. 2009;63(1):39–44.
Gu CJ, Xie F3, Zhang B, Yang HL, Cheng J, He YY, et al. High glucose promotes epithelial-mesenchymal transition of uterus endometrial cancer cells by increasing ER/GLUT4-mediated VEGF secretion. Cell Physiol Biochem. 2018;50(2):706–20.
Mongelli-Sabino BM, Canuto LP, Collares-Buzato CB. Acute and chronic exposure to high levels of glucose modulates tight junction-associated epithelial barrier function in a renal tubular cell line. Life Sci. 2017;188:149–57.
Sobel G, Németh J, Kiss A, Lotz G, Szabó I, Udvarhelyi N, et al. Claudin 1 differentiates endometrioid and serous papillary endometrial adenocarcinoma. Gynecol Oncol. 2006;103(2):591–8.
Szabó I, Kiss A, Schaff Z, Sobel G. Claudins as diagnostic and prognostic markers in gynecological cancer. Histol Histopathol. 2009;24(12):1607–15.
Hoerscher A, Horné F, Dietze R, Berkes E, Oehmke F, Tinneberg HR, et al. Localization of claudin-2 and claudin-3 in eutopic and ectopic endometrium is highly similar. Arch Gynecol Obstet. 2020;301(4):1003–11.
Cruz-Bermúdez A, Vicente-Blanco RJ, Gonzalez-Vioque E, Provencio M, Fernández-Moreno MÁ, Garesse R. Spotlight on the relevance of mtDNA in cancer. Clin Transl Oncol. 2017;19(4):409–18.
Guerra F, Guaragnella N, Arbini AA, Bucci C, Giannattasio S, Moro L. Mitochondrial dysfunction: a novel potential driver of epithelial-to-mesenchymal transition in cancer. Front Oncol. 2017;7:295.
Musicco C, Cormio G, Pesce V, Loizzi V, Cicinelli E, Resta L, et al. Mitochondrial dysfunctions in type I endometrial carcinoma: exploring their role in oncogenesis and tumor progression. Int J Mol Sci. 2018;19(7):E2076.
Yang L, Na CL, Luo S, Wu D, Hogan S, Huang T, et al. The phosphatidylcholine transfer protein stard7 is required for mitochondrial and epithelial cell homeostasis. Sci Rep. 2017;7:46416.
Garmpis N, Damaskos C, Garmpi A, Spartalis E, Kalampokas E, Kalampokas T, et al. Targeting histone deacetylases in endometrial cancer: a paradigm-shifting therapeutic strategy? Eur Rev Med Pharmacol Sci. 2018;22(4):950–60.
Wu Y, Starzinski-Powitz A, Guo SW. Trichostatin A, a histone deacetylase inhibitor, attenuates invasiveness and reactivates E-cadherin expression in immortalized endometriotic cells. Reprod Sci. 2007;14(4):374–82.
Hichino A, Okamoto M, Taga S, Akizuki R, Endo S, Matsunaga T, et al. Down-regulation of claudin-2 expression and proliferation by epigenetic inhibitors in human lung adenocarcinoma A549 cells. J Biol Chem. 2017;292(6):2411–21.
Okimura H, Tatsumi H, Ito F, Yamashita S, Kokabu T, Kitawaki J. Endometrioid carcinoma arising from diaphragmatic endometriosis treated with laparoscopy: a case report. J Obstet Gynaecol Res. 2018;44(5):972–27.
Venugopal S, Anwer S, Szászi K. Claudin-2: roles beyond permeability functions. Int J Mol Sci. 2019;20(22):E5655.
Massagué J. G1 cell-cycle control and cancer. Nature. 2004;432(7015):298–306.
Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12(1):9–18.
Acknowledgments
This work was supported by the Ministry of Education, Culture, Sports, Science, and Technology, and the Ministry of Health, Labour and Welfare of Japan.
Authors’ Contributions Statement
T. Okada, T. Konno and T. Kojima designed and coordinated the study and wrote the main manuscript text. T. Okada, T. Konno and T. Kojima analyzed the data. T. Kohno, H.S., S.S. and T.S. contributed reagents/materials. All authors reviewed the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Statement
The protocol for human study was reviewed and approved by the ethics committee of Sapporo Medical University School of Medicine. Written informed consent was obtained from each patient who participated in the investigation. All experiments were carried out in accordance with the approved guidelines and the Declaration of Helsinki.
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tadahi Okada and Takumi Konno are equal first authors.
Electronic Supplementary Material
ESM 1
(DOC 2883 kb).
Rights and permissions
About this article
Cite this article
Okada, T., Konno, T., Kohno, T. et al. Possibility of Targeting Claudin-2 in Therapy for Human Endometrioid Endometrial Carcinoma. Reprod. Sci. 27, 2092–2103 (2020). https://doi.org/10.1007/s43032-020-00230-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-020-00230-6