Abstract
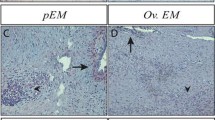
In endometriosis, the lymphatic and immune systems are implicated in disease establishment and progression. The objective of this pilot study was to examine endometrial-like, and for the first time, immune cell populations in lymph nodes associated with deep infiltrating endometriosis (DIE) bowel lesions. Premenopausal women undergoing excision of endometriosis and/or hysterectomy were included. DIE bowel lesion-associated (n = 10) and other pelvic (n = 15) lymph nodes were studied. Samples were immunohistochemically stained for endometrial-like cells (CD10), T cells (CD3, CD4, CD8, and FoxP3), dendritic cells (DC; DC-Lamp and DC-Sign), B cells (CD20, CD79 and plasma), macrophages (CD68), and natural killer cells (NK; CD57). Cell abundance (percentage positive area) and antigen expression (optical density; OD) were quantified. Endometrial-like cells and each immune cell population were present in all studied nodes. The DIE bowel lesion-associated nodes showed features of immune activation, with T cell proliferation (CD3+ area p = 0.007, CD4+ area p = 0.015 compared with other pelvic nodes); and a mixture of helper and regulatory T cells, B cells, DCs, macrophages, and plasma cells present in the paracortex. In DIE bowel lesion-associated compared with other pelvic nodes, CD10+ endometrial-like cells were reduced (percentage positive area p < 0.001, OD p = 0.004). This study provides new insight into lymphatic and immune system involvement in advanced endometriosis. In particular, we have shown evidence of immune activation in DIE lesion-associated nodes. This was despite lower endometrial-like cell numbers compared with other pelvic nodes. The observations contribute to a developing understanding of the local immune response to advanced disease.




Similar content being viewed by others
References
Vinatier D, Cosson M, Dufour P. Is endometriosis an endometrial disease? Eur J Obstet Gynecol Reprod Biol. 2000;91(2):113–25.
Nisolle M, Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril. 1997;68(4):585–96.
Koninckx PR, Martin D. Treatment of deeply infiltrating endometriosis. Curr Opin Obstet Gynecol. 1994;6(3):231–41.
Rossini R, Lisi G, Pesci A, et al. Depth of intestinal wall infiltration and clinical presentation of deep infiltrating endometriosis: evaluation of 553 consecutive cases. J Laparoendosc Adv Surg Tech. 2018;28(2):152–6.
Koninckx PR, Meuleman C, Demeyere S, Lesaffre E, Cornillie FJ. Suggestive evidence that pelvic endometriosis is a progressive disease, whereas deeply infiltrating endometriosis is associated with pelvic pain. Fertil Steril. 1991;55(4):759–65.
Sampson JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol. 1927;3(2):93–109.
Javert CT. Pathogenesis of endometriosis based on endometrial homeoplasia, direct extension, exfoliation and implantation, lymphatic and hematogenous metastasis. Cancer. 1949;2(3):399–410.
Beavis AL, Matsuo K, Grubbs BH, et al. Endometriosis in para-aortic lymph nodes during pregnancy: case report and review of literature. Fertil Steril. 2011;95(7):2429.e2429–13.
Hobbs JE, Bortnick AR. Endometriosis of the lungs: an experimental and clinical study. Am J Obstet Gynecol. 1940;40(5):832–43.
Sarma D, Iyengar P, Marotta TR, TerBrugge KG, Gentili F, Halliday W. Cerebellar endometriosis. Am J Roentgenol. 2004;182(6):1543–6.
Busard MPH, Van Der Houwen LEE, Bleeker MCG, et al. Deep infiltrating endometriosis of the bowel: MR imaging as a method to predict muscular invasion. Abdom Imaging. 2012;37(4):549–57.
Abrao MS, Podgaec S, Dias JA Jr, et al. Deeply infiltrating endometriosis affecting the rectum and lymph nodes. Fertil Steril. 2006;86(3):543–7.
Berbic M, Ng CHM, Black K, et al. A novel pilot study of endometrial stromal cells and immune cell populations in sentinel uterine-draining lymph nodes during the menstrual cycle and in endometriosis. Reprod Sci. 2013;20(11):1339–48.
Mechsner S, Weichbrodt M, Riedlinger WFJ, Bartley J, Kaufmann AM, Schneider A, et al. Estrogen and progestogen receptor positive endometriotic lesions and disseminated cells in pelvic sentinel lymph nodes of patients with deep infiltrating rectovaginal endometriosis: a pilot study. Hum Reprod. 2008;23(10):2202–9.
Mechsner S, Weichbrodt M, Riedlinger WFJ, Kaufmann AM, Schneider A, Kohler C. Immunohistochemical evaluation of endometriotic lesions and disseminated endometriosis-like cells in incidental lymph nodes of patients with endometriosis. Fertil Steril. 2010;94(2):457–63.
Noel JC, Chapron C, Fayt I, Anaf V. Lymph node involvement and lymphovascular invasion in deep infiltrating rectosigmoid endometriosis. Fertil Steril. 2008;89(5):1069–72.
Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75(1):1–10.
Berbic M, Hey-Cunningham AJ, Ng C, Tokushige N, Ganewatta S, Markham R, et al. The role of FoxP3+ regulatory T-cells in endometriosis: a potential controlling mechanism for a complex, chronic immunological condition. Hum Reprod. 2010;25(4):900–7.
Oosterlynck DJ, Cornillie FJ, Waer M, Vandeputte M, Koninckx PR. Women with endometriosis show a defect in natural killer activity resulting in a decreased cytotoxicity to autologous endometrium. Fertil Steril. 1991;56(1):45–51.
Garzetti GG, Ciavattini A, Provinciali M, Fabris N, Cignitti M, Romanini C. Natural killer cell activity in endometriosis: correlation between serum estradiol levels and cytotoxicity. Obstet Gynecol. 1993;81(5 I):665–8.
Schulke L, Berbic M, Manconi F, Tokushige N, Markham R, Fraser IS. Dendritic cell populations in the eutopic and ectopic endometrium of women with endometriosis. Hum Reprod. 2009;24(7):1695–703.
McCluggage WG, Sumathi VP, Maxwell P. CD10 is a sensitive and diagnostically useful immunohistochemical marker of normal endometrial stroma and of endometrial stromal neoplasms. Histopathology. 2001;39(3):273–8.
Dharan M. The adjunctive value of CD10 immunostaining on cell block preparations in pelvic endometriosis. Acta Cytol. 2009;53(6):625–9.
Sharpe-Timms KL. Endometrial anomalies in women with endometriosis. Ann N Y Acad Sci. 2001;943:131–47.
O DF, Roskams T, Van den Eynde K, et al. The presence of endometrial cells in peritoneal fluid of women with and without endometriosis. Reprod Sci. 2017;24(2):242–51.
Junt T, Scandella E, Ludewig B. Form follows function: lymphoid tissue microarchitecture in antimicrobial immune defence. Nat Rev Immunol. 2008;8(10):764–75.
Chantrain CF, DeClerck YA, Groshen S, McNamara G. Computerized quantification of tissue vascularization using high-resolution slide scanning of whole tumor sections. J Histochem Cytochem. 2003;51(2):151–8.
McKay JS, Bigley A, Bell A, Jenkins R, Somers R, Brocklehurst S, et al. A pilot evaluation of the use of tissue microarrays for quantitation of target distribution in drug discovery pathology. Exp Toxicol Pathol. 2006;57(3):181–93.
Tawfik OW, Kimler BF, Davis M, Donahue JK, Persons DL, Fan F, et al. Comparison of immunohistochemistry by automated cellular imaging system (ACIS) versus fluorescence in-situ hybridization in the evaluation of HER-2/neu expression in primary breast carcinoma. Histopathology. 2006;48(3):258–67.
Doudkine A, Macaulay C, Poulin N, Palcic B. Nuclear texture measurements in image cytometry. Pathologica. 1995;87(3):286–99.
Fatemi SH, Earle JA, Stary JM, Lee S, Sedgewick J. Altered levels of the synaptosomal associated protein SNAP-25 in hippocampus of subjects with mood disorders and schizophrenia. NeuroReport. 2001;12(15):3257–62.
Gong Y, Tempfer CB. Regional lymphatic spread in women with pelvic endometriosis. Med Hypotheses. 2011;76(4):560–3.
Coutinho A, Bittencourt LK, Pires CE, et al. MR imaging in deep pelvic endometriosis: a pictorial essay. Radiographics. 2011;31(2):549–67.
Keichel S, Barcena De Arellano ML, Reichelt U, et al. Lymphangiogenesis in deep infiltrating endometriosis. Hum Reprod. 2011;26(10):2713–20.
Borrelli GM, Abrão MS, Taube ET, Darb-Esfahani S, Köhler C, Kaufmann AM, et al. Immunohistochemical investigation of metastasis-related chemokines in deep-infiltrating endometriosis and compromised pelvic sentinel lymph nodes. Reprod Sci. 2015;22(12):1632–42.
Bajénoff M, Breart B, Huang AYC, Qi H, Cazareth J, Braud VM, et al. Natural killer cell behavior in lymph nodes revealed by static and real-time imaging. J Exp Med. 2006;203(3):619–31.
Gasteiger G, Ataide M, Kastenmüller W. Lymph node–an organ for T-cell activation and pathogen defense. Immunol Rev. 2016;271(1):200–20.
Groom JR. Moving to the suburbs: T-cell positioning within lymph nodes during activation and memory. Immunol Cell Biol. 2015;93:330.
Mondino A, Khoruts A, Jenkins MK. The anatomy of T-cell activation and tolerance. Proc Natl Acad Sci U S A. 1996;93(6):2245–52.
Chiang CM, Hill JA. Localization of T cells, interferon-gamma and HLA-DR in eutopic and ectopic human endometrium. Gynecol Obstet Invest. 1997;43(4):245–50.
Ganewatta S, Berbic M, Luscombe GM, Markham R, Fraser IS. The characteristic expression and role of T cell subsets and CD57+ NK cells in different zones of peritoneal endometriotic lesions. J Endometriosis. 2010;2(4):189–96.
Jones RK, Bulmer JN, Searle RF. Phenotypic and functional studies of leukocytes in human endometrium and endometriosis. Hum Reprod Update. 1998;4(5):702–9.
Kempuraj D, Papadopoulou N, Stanford EJ, et al. Increased numbers of activated mast cells in endometriosis lesions positive for corticotropin-releasing hormone and urocortin. Am J Reprod Immunol. 2004;52(4):267–75.
Oosterlynck DJ, Cornillie FJ, Waer M, Koninckx PR. Immunohistochemical characterization of leucocyte subpopulations in endometriotic lesions. Arch Gynecol Obstet. 1993;253(4):197–206.
Mor F, Quintana FJ, Cohen IR. Angiogenesis-inflammation cross-talk: vascular endothelial growth factor is secreted by activated T cells and induces Th1 polarization. J Immunol. 2004;172(7):4618–23.
Zampell JC, Yan A, Elhadad S, Avraham T, Weitman E, Mehrara BJ. CD4+ cells regulate fibrosis and lymphangiogenesis in response to lymphatic fluid stasis. PLoS One. 2012;7(11):e49940.
Antsiferova YS, Sotnikova NY, Posiseeva LV, Shor AL. Changes in the T-helper cytokine profile and in lymphocyte activation at the systemic and local levels in women with endometriosis. Fertil Steril. 2005;84(6):1705–11.
Romagnani S. Type 1 T helper and type 2 T helper cells: functions, regulation and role in protection and disease. Int J Clin Lab Res. 1992;21(2–4):152–8.
Witz CA, Montoya IA, Dey TD, Schenken RS. Characterization of lymphocyte subpopulations and T cell activation in endometriosis. Am J Reprod Immunol. 1994;32(3):173–9.
Yang X, Yang J, Chu Y, et al. T follicular helper cells and regulatory B cells dynamics in systemic lupus erythematosus. PLoS One. 2014;9(2):e88441.
Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4+ T cell immunity. Nat Rev Immunol. 2010;10(4):236–47.
Eifel PJ. Can patients with regional metastases from carcinoma of the endometrium be cured with radiation therapy? Front Radiat Ther Oncol. 1994;28:196–203.
Mais V, Cirronis MG, Piras B, Silvetti E, Cossu E, Melis GB. Intraoperative lymphatic mapping techniques for endometrial cancer. Expert Rev Anticancer Ther. 2011;11(1):83–93.
Netter FH. A compilation of paintings on the normal and pathologic anatomy of the reproductive system, vol. 2. 2nd ed. New York: Ciba Pharmaceutical Company; 1965.
Tanaka H, Sato H, Miura H, Sato N, Fujimoto T, Konishi Y, et al. Can we omit para-aorta lymph node dissection in endometrial cancer? Jpn J Clin Oncol. 2006;36(9):578–81.
Jiang QY, Xia JM, Ding HG, Fei XW, Lin J, Wu RJ. RNAi-mediated blocking of ezrin reduces migration of ectopic endometrial cells in endometriosis. Mol Hum Reprod. 2012;18(9):435–41.
Lessey BA, Metzger DA, Haney AF, McCarty KS Jr. Immunohistochemical analysis of estrogen and progesterone receptors in endometriosis: comparison with normal endometrium during the menstrual cycle and the effect of medical therapy. Fertil Steril. 1989;51(3):409–15.
Shifren JL, Tseng JF, Zaloudek CJ, Ryan IP, Meng YG, Ferrara N, et al. Ovarian steroid regulation of vascular endothelial growth factor in the human endometrium: implications for angiogenesis during the menstrual cycle and in the pathogenesis of endometriosis. J Clin Endocrinol Metab. 1996;81(8):3112–8.
Szuba A, Rockson SG. Lymphedema: anatomy, physiology and pathogenesis. Vasc Med. 1997;2(4):321–6.
Tempfer CB, Wenzl R, Horvat R, et al. Lymphatic spread of endometriosis to pelvic sentinel lymph nodes: a prospective clinical study. Fertil Steril. 2011;96(3):692–6.
Collarino A, Vidal-Sicart S, Perotti G, Valdés Olmos R. The sentinel node approach in gynaecological malignancies. Clin Transl Imaging. 2016;4(5):411–20.
Holloway RW, Abu-Rustum NR, Backes FJ, Boggess JF, Gotlieb WH, Jeffrey Lowery W, et al. Sentinel lymph node mapping and staging in endometrial cancer: a Society of Gynecologic Oncology literature review with consensus recommendations. Gynecol Oncol. 2017;146(2):405–15.
Acknowledgments
The authors thank Professor Ian S. Fraser and Dr. Marina Berbic (Department of Obstetrics and Gynaecology, The University of New South Wales) for the useful comments in the project’s early stage, Dr. Georgina Luscombe (School of Rural Health, The University of Sydney) for the statistical advice, and Dr. Louise Cole (Bosch Institute Advanced Microscopy Facility, The University of Sydney) and Sanaz Maleki (Department of Pathology, The University of Sydney) for the technical support.
Funding
This study was financially supported by the Department of Obstetrics, Gynaecology and Neonatology, The University of Sydney. This study was performed at the Department of Obstetrics, Gynaecology and Neonatology, The University of Sydney.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the Human Research Ethics Committees of the Sydney Local Health District (Royal Prince Alfred Hospital [RPAH] zone; Protocol number X13-00037, HREC/13/RPAH/52) and the University of Sydney (Project number 2013/496).
Conflict of Interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jerman, L.F., Anderson, L., Markham, R. et al. The Lymphatic System in Endometriosis: a Pilot Study of Endometrial-Like Cells and Immune Cell Populations in Lymph Nodes Associated with Deep Infiltrating Bowel Lesions. Reprod. Sci. 27, 977–987 (2020). https://doi.org/10.1007/s43032-020-00171-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-020-00171-0