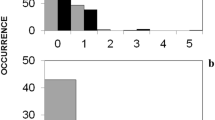
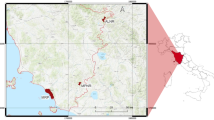
Abstract
Large introduced mammalian herbivores, either as livestock or feral populations, can have important effects on vegetation and animals. Introduced herbivores can be of high conservation concern if they impact threatened native species in protected areas. We evaluated temporal and spatial trends in the relative abundance of introduced water buffalo (Bubalus bubalis) and their potential to induce competition-related ecological and demographic effects on native rusa deer (Rusa timorensis) in Komodo National Park and the Wae Wuul Nature Reserve—two protected areas in Eastern Indonesia. To monitor ungulate population abundances and population growth rates of water buffalo and rusa deer, we counted their dung (a validated abundance index in these two species) along 350 permanently marked transects at 11 sites over five islands annually between 2003 and 2018. Water buffalo dung abundance varied with the site, year and their interaction. Water buffalo dung was most abundant within the Wae Wuul Nature Reserve, and their dung abundance varied considerably among sites in Komodo National Park. Water buffalo site-specific dung abundances fluctuated independently over time. In areas of high water buffalo dung densities, rusa deer altered resource use and had lower population densities than sites without water buffalo. Rusa deer dung-based population growth rates were negatively influenced by density-dependent regulation and only weakly by water buffalo dung density. Currently, managers do not control water buffalo in either protected area. Nevertheless, the benefits of control could reduce water buffalo effects on vegetation composition, watercourse integrity, or the potential to transmit disease into native ungulates.






Similar content being viewed by others
Data accessibility
Data will be deposited in the Dryad data repository (https://datadryad.org/) on acceptance of the manuscript for publication.
References
Acebes P, Traba J, Malo J (2012) Co-occurrence and potential for competition between wild and domestic large herbivores in a South American desert. J Arid Environ 77:39–44
Allen RB, Forsyth DM, Allen RK, Affeld K, MacKenzie DI (2015) Solar radiation determines site occupancy of coexisting tropical and temperate deer species introduced to New Zealand forests. PLoS ONE 10:e0128924
Allred BW, Fuhlendorf SD, Hovick TJ, Dwayne Elmore R, Engle DM, Joern A (2013) Conservation implications of native and introduced ungulates in a changing climate. Glob Change Biol 19:1875–1883. https://doi.org/10.1111/gcb.12183
Anderwald P, Haller RM, Filli F (2016) Heterogeneity in primary productivity influences competitive interactions between red deer and Alpine chamois. PLoS ONE 11:e0146458
Ariefiandy A, Purwandana D, Seno A, Ciofi C, Jessop TS (2013) Can camera traps monitor Komodo dragons a large ectothermic predator? PLoS ONE 8:e58800
Ariefiandy A, Purwandana D, Natali C, Imansyah M, Surahman M, Jessop T, Ciofi C (2015) Conservation of Komodo dragons Varanus komodoensis in the Wae Wuul nature reserve Flores, Indonesia: a multidisciplinary approach. Int Zoo Yearb 49:67–80
Ariefiandy A et al (2016) Temporal and spatial dynamics of insular Rusa deer and wild pig populations in Komodo National Park. J Mammal 97:1652–1662
Ariefiandy A, Purwandana D, Ciofi C, Jessop TS (2020) Komodo survival program: an NGO’s approach to assisting Komodo Dragon conservation and management. In: O’Donnell SC and Walls KM (eds) Strategies for conservation success in herpetology. Society for the Study of Amphibians and Reptiles, University Heights, OH, USA
Auffenberg W (1981) The behavioural ecology of the Komodo monitor. Florida University Press, Gainesville
Bleyhl B et al (2019) Assessing niche overlap between domestic and threatened wild sheep to identify conservation priority areas. Divers Distrib 25:129–141
Braithwaite RW, Dudzinski M, Ridpath M, Parker B (1984) The impact of water buffalo on the monsoon forest ecosystem in Kakadu National Park. Aust J Ecol 9:309–322
Butchart SH et al (2010) Global biodiversity: indicators of recent declines. Science 328:1164–1168
Carlson CJ et al (2019) The global distribution of Bacillus anthracis and associated anthrax risk to humans, livestock, and wildlife. Nature Microbiology 4:1337–1343
Chesson P (2000) Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst 31:343–366. https://doi.org/10.1146/annurev.ecolsys.31.1.343
Clout MN, Russell JC (2008) The invasion ecology of mammals: a global perspective. Wildl Res 35:180–184. https://doi.org/10.1071/WR07091
Clutton-Brock T, Illius A, Wilson K, Grenfell B, MacColl A, Albon S (1997) Stability and instability in ungulate populations: an empirical analysis. Am Nat 149:195–219
Coulson T, Milner-Gulland E, Clutton-Brock T (2000) The relative roles of density and climatic variation on population dynamics and fecundity rates in three contrasting ungulate species. Proc R Soc Lond B Biol Sci 267:1771–1779
Davis MA (2003) Biotic globalization: does competition from introduced species threaten biodiversity? Bioscience 53:481–489
de Boer WF, Prins HHT (1990) Large herbivores that strive mightily but eat and drink as friends. Oecologia 82:264–274. https://doi.org/10.1007/BF00323544
Dolman PM, Wäber K (2008) Ecosystem and competition impacts of introduced deer. Wildl Res 35:202–214
Ferretti F, Fattorini N (2020) Competitor densities, habitat and weather: effects on interspecific interactions between wild deer species. Integr Zool (published online). https://doi.org/10.1111/1749-4877.12470
Ferretti F, Lovari S (2014) Introducing aliens: problems associated with invasive exotics. In: Putman R, Apollonio M (eds) Behaviour and management of European ungulates. Whittles Publishing, Dunbeath, pp 78–109
Ferretti F, Mori E (2020) Displacement interference between wild ungulate species: does it occur? Ethol Ecol Evol 32:2–15
Ferretti F, Sforzi A, Lovari S (2011) Behavioural interference between ungulate species: roe are not on velvet with fallow deer. Behav Ecol Sociobiol 65:875–887
Fleischner TL (1994) Ecological costs of livestock grazing in Western North America. Conserv Biol 8:629–644
Focardi S, Tinelli A (2005) Herbivory in a Mediterranean forest: browsing impact and plant compensation Acta Oecologica-International. J Ecol 28:239–247. https://doi.org/10.1016/j.actao.2005.05.010
Focardi S, Aragno P, Montanaro P, Riga F (2006) Inter-specific competition from fallow deer Dama dama reduces habitat quality for the Italian roe deer Capreolus capreolus italicus. Ecography 29:407–417. https://doi.org/10.1111/j.2006.0906-7590.04442.x
Forsyth DM, Hickling GJ (1998) Increasing Himalayan tahr and decreasing chamois densities in the eastern Southern Alps. New Zealand: evidence for interspecific competition. Oecologia 113:377–382. https://doi.org/10.1007/s004420050389
Forsyth DM, Caley P (2006) Testing the irruptive paradigm of large-herbivore dynamics. Ecology 87:297–303
Francis C (2019) Field guide to the mammals of South-east Asia. Bloomsbury Publishing, London
Freeland W, Boulton W (1990) Feral water buffalo (Bubalus bubalis) in the major floodplains of the 'Top End', Northern Territory, Australia: population growth and the Brucellosis and Tuberculosis Eradication Campaign. Australian Wildlife Research 17:411–420
Fritz H, De Garine-Wichatitsky M, Letessier G (1996) Habitat use by sympatric wild and domestic herbivores in an African savanna woodland: the influence of cattle spatial behaviour. J Appl Ecol 33:589–598
Gordon IJ (2003) Browsing and grazing ruminants: are they different beasts? For Ecol Manag 181:13–21. https://doi.org/10.1016/s0378-1127(03)00124-5
Gordon IJ, Illius AW (1989) Resource partitioning by ungulates on the Isle of Rhum. Oecologia 79:383–389. https://doi.org/10.1007/BF00384318
Hadfield JD (2010) MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J Stat Softw 33:1–22
Hamilton CA (1981) Rusa deer in the Royal National Park: diet, dietary overlap with Wallabia bicolor, influence on the vegetation, distribution and movements. Masters Thesis, University of Sydney.
Holechek JL, Vavra M, Pieper RD (1982) Botanical compostion of determination of range herbivore diets. A review grazing animals, forage resources. Rangel Ecol Manag J Range Manag Arch 35:309–315
Imperio S, Focardi S, Santini G, Provenzale A (2012) Population dynamics in a guild of four Mediterranean ungulates: density-dependence, environmental effects and inter-specific interactions. Oikos 121:1613–1626
Jessop TS, Ariefiandy A, Purwandana D, Benu YJ, Hyatt M, Letnic M (2019) Little to fear: largest lizard predator induces weak defense responses in ungulate prey. Behav Ecol 30:624–36
Jessop TS et al (2020) Komodo dragons are not ecological analogs of apex mammalian predators. Ecology 101:e02970
Joys AC, Fuller RJ, Dolman PM (2004) Influences of deer browsing, coppice history, and standard trees on the growth and development of vegetation structure in coppiced woods in lowland England. For Ecol Manag 202:23–37. https://doi.org/10.1016/j.foreco.2004.06.035
Jung TS, Stotyn SA, Czetwertynski SM (2015) Dietary overlap and potential competition in a dynamic ungulate community in northwestern Canada. J Wildl Manag 79:1277–1285
Keiper RR (1985) Are sika deer responsible for the decline of white-tailed deer on Assateague Island, Maryland? Wildl Soc Bull 1973–2006(13):144–146
Kiddie DG (1962) The Sika Deer (Cervus nippon) in New Zealand. Information Series 44. New Zealand Forest Service: Wellington. New Zealand.
Loft ER, Menke JW, Kie JG (1991) Habitat shifts by mule deer: the influence of cattle grazing. J Wildl Manag 55:16–26
Lovari S, Ferretti F, Corazza M, Minder I, Troiani N, Ferrari C, Saddi A (2014) Unexpected consequences of reintroductions: competition between reintroduced red deer and Apennine chamois. Anim Conserv 17:359–370
Madhusudan M (2004) Recovery of wild large herbivores following livestock decline in a tropical Indian wildlife reserve. J Appl Ecol 41:858–869
Martindah E (2018) Risk factors, attitude and knowledge of farmers in controlling anthrax WARTAZOA Indonesian. Bull Anim Vet Sci 27:135–144
Mishra C, Van Wieren SE, Ketner P, Heitkonig IMA, Prins HHT (2004) Competition between domestic livestock and wild bharal Pseudois nayaur in the Indian Trans-Himalaya. J Appl Ecol 41:344–354. https://doi.org/10.1111/j.0021-8901.2004.00885.x
Nunez MA, Pauchard A (2010) Biological invasions in developing and developed countries: does one model fit all? Biol Invas 12:707–714
Ogutu JO, Owen-Smith N (2003) ENSO, rainfall and temperature influences on extreme population declines among African savanna ungulates. Ecol Lett 6:412–419
Ogutu JO et al (2010) Large herbivore responses to water and settlements in savannas. Ecol Monogr 80:241–266. https://doi.org/10.1890/09-0439.1
Pascual-Rico R, Sánchez-Zapata JA, Navarro J, Eguía S, Anadón JD, Botella F (2020) Ecological niche overlap between co-occurring native and exotic ungulates: insights for a conservation conflict. Biol Invas 22:1–12
Petty AM, Werner PA, Lehmann CE, Riley JE, Banfai DS, Elliott LP (2007) Savanna responses to feral buffalo in Kakadu National Park, Australia. Ecol Monogr 77:441–463
Prins HHT (2000) Competition between wildlife and livestock in Africa. Wildlife Conservation by Sustainable Use (eds H.H.T. Prins, J.G. Grootenhuis & T.T. Dolan), pp. 51–80. Kluwer Academic Publishers, Boston, MA
Prior KM, Adams DC, Klepzig KD, Hulcr J (2018) When does invasive species removal lead to ecological recovery? Implications for management success. Biol Invas 20:267–283
Pudyatmoko S (2017) Free-ranging livestock influence species richness, occupancy, and daily behaviour of wild mammalian species in Baluran National Park, Indonesia. Mammal Biol 86:33–41
Purwandana D et al (2014) Demographic status of Komodo dragons populations in Komodo National Park. Biol Conserv 171:29–35. https://doi.org/10.1016/j.biocon.2014.01.017
Purwandana D et al (2015) Evaluating environmental, demographic and genetic effects on population-level survival in an island endemic. Ecography. https://doi.org/10.1111/ecog.01300
Purwandana D, Ariefiandy A, Imansyah MJ, Seno A, Ciofi C, Letnic M, Jessop TS (2016) Ecological allometries and niche use dynamics across Komodo dragon ontogeny. Sci Nat 103:27
Putman RJ (1996) Competition and resource partitioning in temperate ungulate assemblies, vol 3. Springer, Berlin
Ragotzkie KE, Bailey JA (1991) Desert mule deer use of grazed and ungrazed habitats. Rangel Ecol Manag J Range Manag Arch 44:487–490
Ritchie EG, Martin JK, Johnson CN, Fox BJ (2009) Separating the influences of environment and species interactions on patterns of distribution and abundance: competition between large herbivores. J Anim Ecol 78:724–731
Rout T, Kirkwood R, Sutherland D, Murphy S, McCarthy M (2014) When to declare successful eradication of an invasive predator? Anim Conserv 17:125–132
Setyaningsih CA, Behling H, Saad A, Shumilovskikh L, Sabiham S, Biagioni S (2019) First palaeoecological evidence of buffalo husbandry and rice cultivation in the Kerinci Seblat National Park in Sumatra, Indonesia. Veg Hist Archaeobot 28:591–606
Shea K, Chesson P (2002) Community ecology theory as a framework for biological invasions. Trends Ecol Evol 17:170–176
Simberloff D et al (2013) Impacts of biological invasions: what’s what and the way forward. Trends Ecol Evol 28:58–66
Sinclair ARE (1985) Does interspecific competition or predation shape the African ungulate community? J Anim Ecol 54:899–918. https://doi.org/10.2307/4386
Spear D, Chown SL (2009) Non-indigenous ungulates as a threat to biodiversity. J Zool 279:1–17
Spear D, Foxcroft LC, Bezuidenhout H, McGeoch MA (2013) Human population density explains alien species richness in protected areas. Biol Conserv 159:137–147
Stewart KM, Bowyer RT, Kie JG, Cimon NJ, Johnson BK (2002) Temporospatial distributions of elk, mule deer, and cattle: resource partitioning and competitive displacement. J Mammal 83:229–244
Tulloch D (1969) Home range in feral water buffalo, Bubalus bubalis Lydekker. Aust J Zool 17:143–152
Valeix M, Chamaillé-Jammes S, Fritz H (2007) Interference competition and temporal niche shifts: elephants and herbivore communities at waterholes. Oecologia 153:739–748
Vitousek PM, Mooney HA, Lubchenco J, Melillo JM (1997) Human domination of earth’s ecosystems. Science 277:494–499
Voeten MM, Prins HH (1999) Resource partitioning between sympatric wild and domestic herbivores in the Tarangire region of Tanzania. Oecologia 120:287–294
Wardle DA, Barker GM, Yeates GW, Bonner KI, Ghani A (2001) Introduced browsing mammals in New Zealand natural forests: aboveground and belowground consequences. Ecol Monogr 71:587–614
Werner PA (2005) Impact of feral water buffalo and fire on growth and survival of mature savanna trees: an experimental field study in Kakadu National Park, northern Australia. Austral Ecol 30:625–647
Acknowledgements
We are grateful to the Directorate General of Forest Protection and Nature Conservation for issuing research permits to work in Komodo National Park and Wae Wuul Nature Reserve. Protected area staff and local community volunteers are thanked for their support and enthusiastic involvement in the project. We gratefully acknowledge David M. Forsyth for making useful comments and edits on a draft version of the manuscript. Funding was provided by members of the American and European Association of Zoos and Aquariums.
Author information
Authors and Affiliations
Contributions
TSJ and DPP conceived the study, AA, DP, TSJ, MA, MRP, JM and provided field data. TSJ analysed the data. TSJ, and DPP wrote the manuscript. All authors commented on drafts of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling editor: Juan Carranza.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ariefiandy, A., Purwandana, D., Azmi, M. et al. Invasive water buffalo population trends and competition-related consequences for native rusa deer in eastern Indonesian protected areas. Mamm Biol 101, 917–931 (2021). https://doi.org/10.1007/s42991-021-00161-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42991-021-00161-y