Abstract
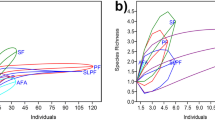
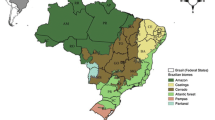
Anthropized environments are associated with loss of biodiversity and have been listed as sites with low species richness for various biological groups. In this study, anuran amphibian species richness, community composition, and taxonomic and functional richness are analyzed for a highly anthropized region of central Mexico, the state of Querétaro, Mexico. A literature and database review found 25 species of anurans recorded in 13 types of environments varying from conserved to anthropized ones. Non-irrigated agricultural environments with annual seasonal harvests, such as submontane scrubland and low deciduous forest, presented the greatest species richness. Environments that had been transformed into crop fields, induced grassland and urban areas showed a greater similarity in their species composition compared to temperate environments without strong modification, i.e., montane cloud forest, pine–oak forest and oak–pine forest. The environments that presented a lower number of species also presented higher values of taxonomic diversity than those that presented the greatest species richness. The study showed that anthropized environments can maintain a different species composition than conserved environments, and maintain greater species and functional richness. This being so, further studies evaluating population density, endemism and conservation status of the species are necessary to evaluate anthropic effect on amphibian communities and other biological groups in the state of Queretaro and in other regions of Mexico.




Similar content being viewed by others
Availability of data and material
Appendix 1, list of scientific collections consulted.
References
Alberti, M., Marzluff, J. M., Shulenberger, E., Bradley, G., Ryan, C., & Zumbrunnen, C. (2003). Integrating humans into ecology: Opportunities and challenges for studying urban ecosystems. BioScience, 53, 1169–1179. https://doi.org/10.1641/0006-3568(2003)053[1169:IHIEOA]2.0.CO;2
Almeida, S. M., Silva, L. C., Cardoso, M. R., Cerqueira, P. V., Juen, L., & Santos, M. P. D. (2016). The effects of oil palm plantations on the functional diversity of Amazonian birds. Journal of Tropical Ecology, 32, 510–525. https://doi.org/10.1017/S0266467416000377
Amori, G., & Luiselli, L. (2013). Null model analyses of small mammal community structure in tropical islands. Tropical Ecology, 54, 23–31.
Andrén, H. (1994). Effects of habitat fragmentation on birds and mammals in landscapes with different proportions of suitable habitat: A review. Oikos, 71, 355–366. https://doi.org/10.2307/3545823
Areendran, G., Rao, P., Raj, K., Mazumdar, S., & Puri, K. (2013). Land use/land cover change dynamics analysis in mining areas of Singrauli district in Madhya Pradesh, India. Tropical Ecology, 54, 239–250.
Aronson, M. F., Nilon, C. H., Lepczyk, C. A., Parker, T. S., Warren, P. S., Cilliers, S. S., Goddard, M. A., Hahs, A. K., Herog, C., Katti, M., La Sorte, F. A., Williams, N. S. G., & Zipperer, W. (2016). Hierarchical filters determine community assembly of urban species pools. Ecology, 97, 2952–2963. https://doi.org/10.1002/ecy.1535
Badoya Celis, A. (2016). El Estado de Querétaro. In R. W. Jones & V. Serrano Cárdenas (Eds.), Historia Natural de Querétaro (pp. 14–23). Universidad Autónoma de Querétaro.
Beebee, T. J. C., & Griffiths, R. A. (2005). The amphibian decline crisis: A watershed for conservation biology? Biological Conservation, 125, 271–285. https://doi.org/10.1016/j.biocon.2005.04.009
Casanoves, F., Pla, L., Di Rienzo, J. A., & Díaz, S. (2010). FDiversity: A software package for the integrated analysis of functional diversity. Methods in Ecology and Evolution, 2, 233–237. https://doi.org/10.1111/j.2041-210X.2010.00082.x
Clark, P. J., Reed, J. M., Tavernia, B. G., Windmiller, B. S., & Regosin, J. V. (2008). Urbanization effects on spotted salamander and wood frog presence and abundance. In J. C. Mitchell, R. E. J. Brown, & B. Bartholomew (Eds.), Urban herpetology (pp. 67–76). Society for the Study of Amphibians and Reptiles, Salt Lake.
Clark, T. W., Warneke, R. M., & George, G. G. (1990). Management and conservation of small populations. In T. W. Clark & J. H. Seebeck (Eds.), The management and conservation of small populations (pp. 1–18). Chicago Zoological Society.
Clarke, K. R., & Gorley, R. N. (2001). PRIMER v5: user manual/tutorial. –PRIMER-E, Plymouth, UK. https://www.primer-e.com/download/. Accessed 15 December 2019.
Clarke, K. R., & Warwick, R. M. (1998). A taxonomic distinctness index and its statistical properties. Journal of Applied Ecology, 35, 523–531. https://doi.org/10.1046/j.1365-2664.1998.3540523.x
Crump, M. L. (2015). Anuran reproductive modes: Evolving perspectives. Journal of Herpetology, 49, 1–16. https://doi.org/10.1670/14-097
Cruz-Elizalde, R., Ramírez-Bautista, A., Hernández-Salinas, U., Berriozabal-Islas, C., & Wilson, L. D. (2019). An updated checklist of the herpetofauna of Querétaro, Mexico: species richness, diversity, and conservation status. Zootaxa, 4638, 273–290. https://doi.org/10.11646/zootaxa.4638.2.7
Cruz-Elizalde, R., Berriozabal-Islas, C., Hernández-Salinas, U., Martínez-Morales, M. A., & Ramírez-Bautista, A. (2016a). Amphibian species richness and diversity in a modified tropical environment of central Mexico. Tropical Ecology, 57, 407–417.
Cruz-Elizalde, R., Padilla García, U., Cruz Pérez, M. S., & Tinoco Navarro, C. (2016b). Herpetofauna del Estado de Querétaro. In R. W. Jones & V. Serrano Cárdenas (Eds.), Historia Natural de Querétaro (pp. 300–319). Universidad Autónoma de Querétaro.
Dayton, G. H., Jung, T. E., & Droege, S. (2004). Large-scale habitat associations of four desert anurans in Big Bend National Park, Texas. Journal of Herpetology, 38, 619–627. https://doi.org/10.1670/125-04N
Dickman, C. R. (1987). Habitat fragmentation and vertebrate species richness in an urban environment. Journal of Applied Ecology, 24, 337–351. https://doi.org/10.2307/2403879
Dixon, J. R., & Lemos-Espinal, J. A. (2010). Anfibios y reptiles del estado de Querétaro. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, México, DF.
DOF (2010) Diario Oficial de la Federación, Norma Oficial Mexicana NOM-059-SEMARNAT-2010. Protección ambiental-Especies nativas de México de flora y fauna silvestres-Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio-lista de especies en riesgo, Second edition.
Duellman, W. E. (2001). Hylid frogs of Middle America. Society for the Study of Amphibians and Reptiles.
Duellman, W. E., & Trueb, L. (1994). Biology of amphibians. The Johns Hopkins University Press.
Environmental Systems Research Institute (ESRI) ArcGIS (2013) Descktop Help. Version 10. Redlands, California, USA. https://www.esri.com/es-es/home. Accessed 1 December 2019.
Ernst, R., Linsenmair, K. E., & Rödel, M.-O. (2006). Diversity erosion beyond the species level: Dramatic loss of functional diversity after selective logging in two tropical amphibian communities. Biological Conservation, 133, 143–155. https://doi.org/10.1016/j.biocon.2006.05.028
Esteller, M. V., & Díaz-Delgado, C. (2002). Environmental effects of aquifer overexploitation: A case of study in the highlands of Mexico. Environmental Management, 29, 266–278. https://doi.org/10.1007/s00267-001-0024-0
Flores-Villela, O., Canseco-Márquez, L., Ochoa-Ochoa, L. M. (2010). Geographic distribution and conservation of the Mexican central highlands herpetofauna. In L. D. Wilson, J. H. Townsend, & J. D. Johnson (Eds.), Conservation of the mesoamerican amphibians and reptiles (pp. 303–321). Utah, USA: Eagle Mountain Publisher, L.C. Eagle Mountain.
Frost, D.R. (2021) Amphibian Species of the World: An Online Reference. Versions 6.0. Available from: http://www.research.amnh.org/herpetology/amphibia/index.html. Accessed 13 February 2021.
GBIF.org (2019) Nieto-Montes de Oca A (2018). Anfibios y reptiles del estado de Querétaro. Comisión nacional para el conocimiento y uso de la biodiversidad. Occurrence dataset https://doi.org/10.15468/xwpedz accessed via GBIF.org on 2019–12–02.
Haila, Y. (2002). A conceptual genealogy of fragmentation research: From island biogeography to landscape ecology. Ecological Applications, 12, 321–334. https://doi.org/10.1890/1051-0761(2002)012[0321:ACGOFR]2.0.CO;2
Huey, R. B., Deutsch, C. A., Tewksbury, J. J., Vitt, L. J., Hertz, P. E., Pérez, H. J. A., & Garland, T. (2009). Why tropical forest lizards are vulnerable to climate warming. Proceedings of the Biological Sciences, 276, 1939–1948. https://doi.org/10.1098/rspb.2008.1957
INEGI. (2017). Instituto nacional de estadística, geografía e informática. México. http://www.inegi.org.mx/inegi/default.aspx. Accessed 12 December 2019.
IUCN (2021). The IUCN red list of threatened species. Version 2016–3. http://www.iucnredlist.org. Accessed 15 January 2021.
Jones, R. W., & Serrano Cárdenas, V. (2016). Historia Natural de Querétaro. Universidad Autónoma de Querétaro.
Kerr, J. T., & Deguise, I. (2004). Habitat loss and the limits to endangered species recovery. Ecology Letters, 7, 1163–1169. https://doi.org/10.1111/j.1461-0248.2004.00676.x
Koleff, P., Gaston, K. J., & Lennon, J. K. (2003). Measuring beta diversity for presence absence data. Journal of Animal Ecology, 72, 367–382. https://doi.org/10.1046/j.1365-2656.2003.00710.x
Laliberté, E., & Legendre, P. (2010). A distance-based framework for measuring functional diversity from multiple traits. Ecology, 91, 299–305. https://doi.org/10.1890/08-2244.1
Laurance, W. F., & Bierregaard, R. O., Jr. (1997). Tropical forest remnants. Ecology, management, and conservation of fragmented communities. Chicago: The University of Chicago Press.
Laurance, W. F., Lovejoy, T. E., Vasconcelos, H. L., Bruna, E. M., Didham, R. K., Stouffer, P. C., Gascon, C., Bierregaard, R. O., Laurance, S. G., & Sampaio, E. (2002). Ecosystem decay of Amazonian forest fragments: A 22-year investigation. Conservation Biology, 16, 605–618. https://doi.org/10.1046/j.1523-1739.2002.01025.x
Lieberman, S. S. (1986). Ecology of the leaf litter herpetofauna of a Neotropical rain forest: La Selva, Costa Rica. Acta Zoologica Mexicana (n.s.), 15, 1–72.
Lips, K. R. (1998). Decline of a tropical montane amphibian fauna. Conservation Biology, 12, 106–117. https://doi.org/10.1111/j.1523-1739.1998.96359.x
Luja, V. H., López, J. A., Cruz-Elizalde, R., & Ramírez-Bautista, A. (2017). Herpetofauna inside and outside from a natural protected area: The case of Reserva Estatal de la Biósfera Sierra San Juan, Nayarit, Mexico. Nature Conservation, 21, 15–38. https://doi.org/10.3897/natureconservation.21.12875
Mason, N. W. H., Mouillot, D., Lee, W. G., & Wilson, J. B. (2005). Functional richness, functional evenness and functional divergence: The primary components of functional diversity. Oikos, 111, 112–118. https://doi.org/10.1111/j.0030-1299.2005.13886.x
Moreno, C. E., Castillo-Campos, G., & Verdú, J. R. (2009). Taxonomic diversity as complementary information to assess plant species diversity in secondary vegetation and primary tropical deciduous forest. Journal of Vegetation Science, 20, 935–943. https://doi.org/10.1111/j.1654-1103.2009.01094.x
Parra-Olea, G., Flores-Villela, O., & Mendoza-Almeralla, C. (2014). Biodiversidad de anfibios en México. Revista Mexicana de Biodiversidad, 85, S460–S466. https://doi.org/10.7550/rmb.32027
Pereyra, L. C., Akmentins, M. S., Salica, M. J., Quiroga, M. F., Moreno, C. E., & Vaira, M. (2021). Tolerant and avoiders in an urban landscape: Anuran species richness and functional groups responses in the Yungas’ forest of NW Argentina. Urban Ecosystem, 24, 141–152. https://doi.org/10.1007/s11252-020-01025-y
Pereyra, L. C., Akmentins, M. S., Vaira, M., & Moreno, C. E. (2018). Disentangling the multiple components of anuran diversity associated to different land-uses in Yungas forests, Argentina. Animal Conservation, 21, 396–404. https://doi.org/10.1111/acv.12406
Pineda, E., & Halffter, G. (2004). Species diversity and habitat fragmentation: Frogs in a tropical montane landscape in México. Biological Conservation, 117, 499–508. https://doi.org/10.1016/j.biocon.2003.08.009
Pineda, E., Moreno, C., Escobar, F., & Halffter, G. (2005). Frog, bat, and dung beetle diversity in the cloud Forest and coffee agroecosystems of Veracruz, México. Conservation Biology, 19, 400–410. https://doi.org/10.1111/j.1523-1739.2005.00531.x
Riemann, J. C., Ndriantsoa, S. H., Rödel, M.-O., & Glos, J. (2017). Functional diversity in a fragmented landscape—Habitat alterations affect functional trait composition of frog assemblages in Madagascar. Global Ecology and Conservation, 10, 173–183. https://doi.org/10.1016/j.gecco.2017.03.005
Rothermel, B. B., & Semlitsch, R. D. (2002). An experimental investigation of landscape resistance of forest versus old-field habitats to emigrating juvenile amphibians. Conservation Biology, 16, 1324–1332. https://doi.org/10.1046/j.1523-1739.2002.01085.x
Saunders, D. A., Hobbs, R. J., & Margules, C. R. (1991). Biological consequences of ecosystem fragmentation: A review. Conservation Biology, 5, 18–32. https://doi.org/10.1111/j.1523-1739.1991.tb00384.x
Schalk, C. M., Montaña, C. G., & Springer, L. (2015). Morphological diversity and community organization of desert anurans. Journal of Arid Environments, 122, 132–140. https://doi.org/10.1016/j.jaridenv.2015.06.019
Schelhas, J., & Greenberg, R. (1996). Forest patches in tropical landscapes. Island Press.
Simon, J. A., Snodgrass, J. W., Casey, R. E., & Sparling, D. W. (2009). Spatial correlates of amphibian use of constructed wetlands in an urban landscape. Landscape Ecology, 24, 361–373. https://doi.org/10.1007/s10980-008-9311-y
Sodhi, N., Bickford, D., Diesmos, A., Lee, T., Koh, L., Brook, B. W., Sekercioglu, C., & Bradshaw, C. J. A. (2008). Measuring the meltdown: drivers of global amphibian extinction and decline. PLoS ONE, 3(e1636), 16. https://doi.org/10.1371/journal.pone.0001636
Stuart, N. S., Chanson, J. S., Cox, N. A., Young, B. E., Rodrigues, A. S. L., Fischman, D. L., & Waller, R. W. (2004). Status and trends of amphibian declines and extinction worldwide. Science, 360, 1783–1786. https://doi.org/10.1126/science.1103538
Villéger, S., Mason, N. W., & Mouillot, D. (2008). New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology, 89, 2290–2301. https://doi.org/10.1890/07-1206.1
Vitt, L. J., & Caldwell, J. P. (2001). The effects of logging on reptiles and amphibians of tropical forests. In R. A. Fimbel, A. Grajal, & J. Robinson (Eds.), The cutting edge (pp. 239–260). Columbia University Press.
Warwick, R. M., & Clarke, K. R. (1995). New biodiversity measures reveal a decrease in taxonomic distinctness with increasing stress. Marine Ecology Progress Series, 129, 301–305.
Warwick, R. M., & Clarke, K. R. (2001). Practical measures of marine biodiversity based on relatedness of species. Oceanography and Marine Biology: an Annual Review, 39, 207–231.
Weiher, E. (2011). A primer of trait and functional diversity. In A. E. Magurran & B. J. McGill (Eds.), Biological diversity: Frontiers in measurement and assessment (pp. 175–193). Oxford University Press.
Wells, K. D. (2007). The ecology and behavior of amphibians. The University of Chicago Press.
Whittaker, R. H. (1972). Evolution and measurement of species diversity. Taxon, 21, 213–251. https://doi.org/10.2307/1218190
Wiens, J. J., & Donoghue, M. J. (2004). Historical biogeography, ecology and species richness. Trends in Ecology & Evolution, 19, 639–644. https://doi.org/10.1016/j.tree.2004.09.011
Wilson, L. D., Johnson, J. D., & Mata-Silva, V. (2013). A conservation reassessment of the amphibians of Mexico based on the EVS measure. Amphibian & Reptile Conservation, 7, 97–127.
Acknowledgements
The authors acknowledge the support of the Consejo Nacional de Ciencia y Tecnología through the Project CONACyT #271845, “Red Temática Biología, Manejo y Conservación de Fauna Nativa en Ambientes Antropizados”. We thank the logistic support to PRODEP through Cuerpo Académico de Ecología y Diversidad Faunística, Universidad Autónoma de Querétaro. We thank Margaret Schroeder for her revising the language of our manuscript.
Author information
Authors and Affiliations
Contributions
RCE and RPL conceived the idea. RCE and CBI analyzed the data. RCE wrote the paper. ARB reviewed the final version of the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
All co-authors agree.
Consent for publication
All co-authors agree.
Appendix 1 National and foreign collections consulted with records of amphibians species from Querétaro, Mexico.
Appendix 1 National and foreign collections consulted with records of amphibians species from Querétaro, Mexico.
Collection | Country |
|---|---|
Colección Herpetológica Centro de Investigaciones Biológicas, UAEH | Mexico |
Comisión Nacional para el Conocimiento y Uso de la Biodiversidad CONABIO | Mexico |
Colección Nacional de Anfibios y Reptiles de la Facultad de Ciencias del Instituto de Biología, UNAM | Mexico |
Museo de Zoología de la Facultad de Ciencias de la Universidad Nacional Autónoma de México MZFC | Mexico |
Collection of Vertebrates, University of Texas at Arlintong UTA | USA |
Collection of Herpetology, University of California at Berkeley Museum of Vertebrate Zoology MVZ | USA |
Collection Herpetology, Texas Cooperative Wildlife Collection, Texas A and M University TCWC | USA |
Collection Herpetology, Oklahoma Museum of Natural History University of Oklahoma OMNH | USA |
Collection of Herpetology, Zoology Section of Los Angeles Country Museum of Natural History LACM | USA |
Collection of Herpetology, University of Illinois Museum of Natural History UIMNH | USA |
Collection of Herpetology, Museum of Comparative Zoology, Harvard University MCZ | USA |
The University of Michigan Museum of Zoology UMMZ | USA |
Comisión Nacional para el Conocimiento y Uso de la Biodiversidad CONABIO | México |
Rights and permissions
About this article
Cite this article
Cruz-Elizalde, R., Pineda-López, R., Ramírez-Bautista, A. et al. Diversity and composition of anuran communities in transformed landscapes in central Mexico. COMMUNITY ECOLOGY 23, 103–114 (2022). https://doi.org/10.1007/s42974-022-00076-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42974-022-00076-9