Abstract
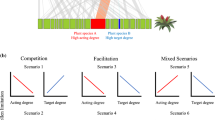
Pollinators can drive indirect facilitative and competitive indirect interactions among plant species. Most work on indirect facilitation via pollinators has focused on “magnet species” which enhance the pollination success of their neighbors because they are disproportionately attractive. However, focusing on magnet species may overestimate the generality of indirect facilitation and underestimate the occurrence of indirect competition among plant species via pollinators. We used experimental arrangements that included three flowering native intermountain prairie species (Achillea millefolium, Gaillardia aristata, and Linum lewisii), all of which are similarly attractive to pollinators, to explore how variation in species richness and density affected pollinator visitation rates, diversity, and behavior. All three plant species experienced significant increases in pollinator visitation and the species richness of visiting pollinator communities when grown with another species that was in flower at the same time. This “diversity” effect was stronger than the effects of the total density of individual plants in flower in a plot. We also found an increase in visitation time, per flower, for solitary pollinator species in plots with two species in flower compared to plots with one plant species in flower. Social pollinator species did not increase visitation time in two-species plots. Finally, seed set by Linum was significantly greater in two-species than in one-species plots. Our results indicate that indirect facilitative interactions mediated by pollinators may be common in intermountain prairie plant communities and that such indirect interactions do not have to mediated by benefactor species that are strikingly more attractive than their beneficiaries.




Similar content being viewed by others
Availability of data and material
Data will be available at https://www.mtnsfepscor.org/.
Code availability
Not applicable.
References
Alaux, C., Ducloz, F., Crauser, D., & Le Conte, T. (2010). Diet effects on honeybee immunocompetence. tp://rsbl.royalsocietypublishing.org/content/early/2010/01/18/rsbl.2009.0986.short.
Albor, C., García-Franco, J. G., Parra-Tabla, V., Díaz-Castelazo, C., & Arceo-Gómez, G. (2019). Taxonomic and functional diversity of the co-flowering community differentially affect Cakile edentula pollination at different spatial scales. Journal of Ecology, 107, 2167–2181. https://doi.org/10.1111/1365-2745.13183
Albrecht, M., Ramis, M. R., & Traveset, A. (2016). Pollinator-mediated impacts of alien invasive plantson the pollination of native plants: The role of spatial scale and distinct behaviour among pollinator guilds. Biological Invasions, 18, 1801–1812. https://doi.org/10.1007/s10530-016-1121-6
Borges, R. M., Gowda, V., & Zacharias, M. (2003). Butterfly pollination and high-contrast visual signalsin a low-density distylous plant. Oecologia, 136, 571–573. https://doi.org/10.1007/s00442-003-1336-y
Braun, J., & Lortie, C. J. (2019). Finding the bees knees: A conceptual framework and systematicreview of the mechanisms of pollinator-mediated facilitation. Perspectives in Plant Ecology, Evolution and Systematics, 36, 33–40. https://doi.org/10.1016/j.ppees.2018.12.003
Coffey, M. F., & Breen, J. (1997). Seasonal variation in pollen and nectar sources of honey beesin Ireland. Journal of Apicultural Research, 36, 63–76.
Crane, E. (1975). Honey. A comprehensive survey. William Heinemann Ltd.
Crane, E. (1990). Bees and beekeeping: Science. William Heinemann Ltd.
Dauber, J., Biesmeijer, J. C., Gabriel, D., Kunin, W. E., Lamborn, E., Meyer, B., & Settele, J. (2010). Effects of patch size and density on flower visitation and seed set of wild plants: A Pan-European approach. Journal of Ecology, 98, 188–196. https://doi.org/10.1111/j.1365-2745.2009.01590.x
Filho, LC., & Packer, L. (2015). Revision of the Neotropical subgenera Coelioxys (Platycoelioxys) Mitchell and C. (Rhinocoelioxys) Mitchell (Hymenoptera; Megachilidae) with the description of one new species. Zootaxa, 3941(2), 151–203. https://doi.org/10.11646/zootaxa.3941.2.1
De Groot, A. P. (1953). Protein and amino acid requirements of the honeybee. Physiologia Comparata Et Oecologia, 3, 1–90.
Dornhaus, A., Klügl, F., Oechslein, C., Puppe, F., & Chittka, L. (2006). Benefits of recruitment in honey bees: Effects of ecology and colony size in an individual-based model. Behavioral Ecology, 17, 336–344. https://doi.org/10.1093/beheco/arj036
Feldman, T. S., Morris, W. F., & Wilson, W. G. (2004). When can two plant species facilitate each other’s pollination? Oikos, 105, 197–207. https://doi.org/10.1111/j.0030-1299.2004.12845.x
Fort, H., Vázquez, D. P., & Lan, B. L. (2016). Abundance and generalisation in mutualistic networks: Solving the chicken-and-egg dilemma. Ecology Letters, 19, 4–11.
Galetto, L., & Bernardello, G. (2003). Nectar sugar composition in angiosperms from Chaco and Patagonia (Argentina): An animal visitor’s matter? Plant Systematics and Evolution, 238, 69–86. https://doi.org/10.1007/s00606-002-0269-y
Garibaldi, L. A., Steffan-Dewenter, I., Winfree, R., Aizen, M. A., Bommarco, R., Cunningham, S. A., & Bartomeus, I. (2013). Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science, 339, 1608–1611. https://doi.org/10.1126/science.1230200
Ghazoul, J. (2006). Floral diversity and the facilitation of pollination. Journal of Ecology, 94, 295–304.
Goulson, D., Ollerton, J., & Sluman, C. (1997). Foraging strategies in the small skipper butterfly, Thymelicus flavus: When to switch? Animal Behaviour, 53, 1009–1016.
Goulson, D. (1999). Foraging strategies of insects for gathering nectar and pollen, andimplications for plant ecology and evolution. Perspectives in Plant Ecology, Evolution and Systematics, 2, 185–209.
Goulson, D. (2003). Effects of introduced bees on native ecosystems. Annual Review of Ecology, Evolution, and Systematics, 34, 1–26.
Heinrich, B. (1976). Bumblebee foraging and the economics of sociality: How have bumblebeesevolved to use a large variety of flowers efficiently? American Scientist, 64, 384–395.
Hegland, S. J., & Boeke, L. (2006). Relationships between the density and diversity of floral resourcesand flower visitor activity in a temperate grassland community. Ecological Entomology, 31, 532–538. https://doi.org/10.1111/j.1365-2311.2006.00812.x
Herbert, E. W., Bickley, W. E., & Shimanuki, H. (1970). The brood-rearing capability of caged honeybees fed dandelion and mixed pollen diets. Journal of Economic Entomology, 63, 215–218.
Hingston, A. B., & McQuillan, P. B. (1998). Nectar robbing in Epacris impressa (Epacridaceae) by therecently introduced bumblebee Bombus terrestris (Apidae) in Tasmania. Victorian Naturalist, 115, 116–119.
Jennersten, O. L. A. (1988). Pollination in Dianthus deltoides (Caryophyllaceae): Effects of habitat fragmentation on visitation and seed set. Conservation Biology, 2, 359–366.
Johnson, L. K., & Hubbell, S. P. (1974). Aggression and competition among stingless bees: Field studies. Ecology, 55, 120–127. https://doi.org/10.2307/1934624
Johnson, S. D., Peter, C. I., Nilsson, L. A., & Ågren, J. (2000). Pollination success in a deceptive orchid is enhanced by co-occurring rewarding magnet plants. Ecology, 84, 2919–2927. https://doi.org/10.1890/02-0471
Lara-Romero, C., Seguí, J., Pérez-Delgado, A., Nogales, A., & Traveset, A. (2019). Beta diversity and specialization in plant–pollinator networks along an elevational gradient. Journal of Biogeography, 46, 1598–1610. https://doi.org/10.1111/jbi.13615
Laverty, T. M., & Plowright, R. C. (1988). Fruit and seed set in mayapple (Podophyllum peltatum): Influence of intraspecific factors and local enhancement near Pedicularis canadensis. Canadian Journal of Botany, 66, 173–178. https://doi.org/10.1139/b88-027
Laverty, T. M. (1992). Plant interactions for pollinator visits: A test of the magnet species effect. Oecologia, 89, 502–508.
Lázaro, A., Lundgren, R., & Totland, O. (2009). Co-flowering neighbors influence the diversity and identity of pollinator groups visiting plant species. Oikos, 118, 691–702. https://doi.org/10.1111/j.1600-0706.2008.17168.x
Levin, D. A., & Anderson, W. W. (1970). Competition for pollinators between simultaneously flowering species. The American Naturalist, 104, 455–467. https://doi.org/10.1086/282680
Liao, H., Luo, W., Peng, S., & Callaway, R. M. (2015). Plant diversity, soil biota and resistance to exotic invasion. Diversity and Distributions, 21, 826–835. https://doi.org/10.1111/ddi.12319
Macfarlane, R. P. (1974). Ecology of Bombinae (Hymenoptera: Apidae) of Southern Ontario, with emphasis on their natural enemies and relationships with flowers. PhD, thesis, University of Guelph, Guelph, ON, Canada.
Masters, J. A., & Emery, S. M. (2015). The showy invasive plant Ranunculus ficaria facilitates pollinator activity, pollen deposition, but not always seed production for two native spring ephemeral plants. Biological Invasions, 17, 2329–2337. https://doi.org/10.1007/s10530-015-0878-3
Mevi-Schütz, J., & Erhardt, A. (2005). Amino acids in nectar enhance butterfly fecundity: A long-awaited link. The American Naturalist, 165, 411–419. https://doi.org/10.1086/429150
Moeller, D. A. (2004). Facilitative interactions among plants via shared pollinators. Ecology, 85, 3289–3301. https://doi.org/10.1890/03-0810
Molina-Montenegro, M. A., Badano, E. I., & Cavieres, L. A. (2008). Positive interactions among plantspecies for pollinator service: Assessing the ‘magnet species’ concept with invasive species. Oikos, 117, 1833–1839. https://doi.org/10.1111/j.0030-1299.2008.16896.x
Muñoz, A. A., & Cavieres, L. A. (2008). The presence of a showy invasive plant disrupts pollinatorservice and reproductive output in native alpine species only at high densities. Journal of Ecology, 96, 459–467. https://doi.org/10.1111/j.1365-2745.2008.01361.x
Nagamitsu, T., & Inoue, T. (1997). Aggressive foraging of social bees as a mechanism of floral resource partitioning in an Asian tropical rainforest. Oecologia, 110, 432–439.
Nieh, J. C., Contrera, F. A., Ramírez, S., & Imperatriz-Fonseca, V. L. (2003). Variation in the ability to communicate three-dimensional resource location by stingless bees from different habitats. Animal Behaviour, 66, 1129–1139. https://doi.org/10.1006/anbe.2003.2289
Petanidou, T., Van Laere, A., Ellis, W. N., & Smets, E. (2006). What shapes amino acid and sugarcomposition in Mediterranean floral nectars? Oikos, 115, 155–169. https://doi.org/10.1111/j.2006.0030-1299.14487.x
Platt, W. J., Hill, G. R., & Clark, S. (1974). Seed production in a prairie legume (Astragalus canadensis L.). Oecologia, 17, 55–63.
Rathcke, B. (1983). Competition and facilitation among plants for pollination. In L. Real (Ed.), Pollination biology (pp. 305–329). Academic Press Inc.
Rathcke, B. (1988). Interactions for pollination among coflowering shrubs. Ecology, 69, 446–457. https://doi.org/10.2307/1940443
Reader, R. J. (1975). Competitive relationships of some bog ericads for major insectpollinators. Canadian Journal of Botany, 53, 1300–1305. https://doi.org/10.1139/b75-156
Schemske, D. W. (1981). Floral convergence and pollinator sharing in two bee-pollinated tropical herbs. Ecology, 62, 946–954. https://doi.org/10.2307/1936993
Sih, A., & Baltus, M. S. (1987). Patch size, pollinator behavior, and pollinator limitation in catnip. Ecology, 68, 1679–1690. https://doi.org/10.2307/1939860
Szczêsna, T. (2006). Protein content and amino acid composition of bee-collected pollen from selected botanical origins. Journal of Apicultural Science, 50, 81–90.
Underwood, N., Hambäck, P. A., & Inoye, B. D. (2020). Pollinators, herbivores, and plant neighbourhood effects. The Quarterly Review of Biology, 95, 37–57.
van der Plas, F. (2019). Biodiversity and ecosystem functioning in naturally assembled communities. Biological Reviews, 94, 1220–1245. https://doi.org/10.1111/brv.12499
Vázquez, D. P., & Aizen, M. A. (2003). Null model analyses of specialization in plant–pollinator interactions. Ecology, 84, 2493–2501.
Walther, J. B., Van Der Heide, B., Kim, S.-Y., Westerman, D., & Tong, S. T. (2008). The role of friends’ appearance and behavior on evaluations of individuals on Facebook: Are we known bythe company we keep? Human Communication Research, 34, 28–49.
Waser, N. M., & Fugate, M. L. (1986). Pollen precedence and stigma closure: A mechanism ofcompetition for pollination between Delphinium nelsonii and Ipomopsis aggregata. Oecologia, 70, 73–577. https://doi.org/10.1007/BF00379906
Williams, N. M., & Tepedino, V. J. (2003). Consistent mixing of near and distant resources in foragingbouts by the solitary mason bee Osmia lignaria. Behavioral Ecology, 14, 141–149. https://doi.org/10.1093/beheco/14.1.141
Ye, Z.-M., Dai, W.-K., Jin, X.-F., Gituru, R. W., Wang, Q. F., & Yang, C.-F. (2013). Competition and facilitation amongplants for pollination: Can pollinator abundance shift the plant–plant interactions? Plant Ecology, 215, 3–13.
Acknowledgements
We are grateful for assistance from Amber and Isabella Maccarone, and their unfailing support of SD and this research. This research was made possible by the generous donations of John and Dian Adams whose love of insect pollinators is surpassed only by their support of me and this research. RMC acknowledges support from NSF EPSCoR Track-1 EPS-1101342 (INSTEP 3) and NSF EPSCoR Cooperative Agreement OIA-1757351.
Funding
John and Diane Adams donations; NSF EPSCoR Track-1 EPS-1101342 (INSTEP 3); NSF EPSCoR Cooperative Agreement OIA-1757351.
Author information
Authors and Affiliations
Contributions
SD conceived of and developed the project, SD and RMC collected data, HL developed the common gardens and conducted statistics, and SD and RMC wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Ethical approval
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Debnam, S., Liao, H. & Callaway, R.M. Indirect facilitation mediated by pollinators in intermountain prairie. COMMUNITY ECOLOGY 22, 309–317 (2021). https://doi.org/10.1007/s42974-021-00056-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42974-021-00056-5