Abstract
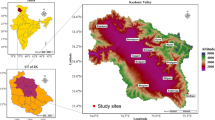
Treelines and treeline species are being studied the world over because of their sensitivity to climate change but most of these studies have not focused on the essential belowground mutualists which could influence the outcome of the interaction between treeline species and climate change, such as the treeline shift. To fill this knowledge gap, we processed 27 root samples of Betula utilis from two sites in the Kashmir Himalaya and identified 590 species of root-associated fungi belonging to 158 genera, 80 families, 38 orders, 15 classes and 3 phyla from a total of 9157 quality reads. Most of the identified species belonged to Basidiomycetes. Papiliotrema, Humaria, Sphaerobolus, Sebicina, Inocybe, Cryptococcus, Lactarius and Laccaria were the dominant taxa. Symbiotroph, Saproptroph-Symbiotroph and Saprotrophs were the dominant trophic modes of the identified root-associated fungi and most of the identified species belonged to the Ectomycorrhizae (ECM) guild. Differences in the species richness between the two sites were noted and a total of 511 taxa were recovered from root samples at the Sinthan Top site in south Kashmir whereas only 302 taxa were recovered from root samples at the Apharwat site in north Kashmir. Papiliotrema and Inocybe with > 75% prevalence represented the core microbiome. Our study provides the first detailed and comprehensive account of the diversity of fungi associated with the roots of B. utilis and paves way for exploring their functional role in the growth of this treeline species, particularly under changing climate.









Similar content being viewed by others
Data availability
The sequence data generated for this study is available at the NCBI sequence read archive under the accession number PRJNA669364. For identification of plant species, a voucher specimen has been submitted to the host University’s herbarium (Kashmir University Botanical Herbarium) under voucher specimen number: 3714-KASH.
Change history
30 May 2022
A Correction to this paper has been published: https://doi.org/10.1007/s42965-022-00249-7
References
Agerer R, Hartmann A, Pritsch K, Raidl S, Schloter M, Verma R, Weigt R (2012) Plants and their ectomycorrhizosphere: cost and benefit of symbiotic soil organisms. Growth Def Plants 220:213–242
Ahonen SH, Ylänne H, Väisänen M, Ruotsalainen AL, Männistö MK, Markkola A, Stark S (2021) Reindeer grazing history determines the responses of subarctic soil fungal communities to warming and fertilization. New Phytol 232(2):788–801
Al-Shammari TA, Bahkali AH, Elgorban AM, El-Kahky MT, Al-Sum BA (2013) The use of Trichoderma longibrachiatum and Mortierella alpina against root-knot nematode, Meloidogyne javanica on tomato. J Pure Appl Microbiol 7:199–207
Antony R, Sanyal A, Kapse N, Dhakephalkar PK, Thamban M, Nair S (2016) Microbial communities associated with Antarctic snow pack and their biogeochemical implications. Microbiol Res 192:192–202
Ashburner K, McAllister H (2013) Botanical Magazine Monograph: The genus Betula: a taxonomic revision of birches. Published by royal botanic gardens, United Kingdom
Assad R, Reshi ZA, Rashid I, Wali D, Bashir I, Rafiq I (2021) Metabarcoding of root-associated ectomycorrhizal fungi of Himalayan pindrow fir through morphotyping and Next Generation Sequencing. Trees for People 6:100153
Baldrian P (2008) Wood-inhabiting ligninolytic basidiomycetes in soils: ecology and constraints for applicability in bioremediation. Fungal Ecol 1(1):4–12
Bardgett RD, Van Der Putten WH (2014) Belowground biodiversity and ecosystem functioning. Nature 515(7528):505–511
Bennett AE, Classen AT (2020) Climate change influences mycorrhizal fungal–plant interactions, but conclusions are limited by geographical study bias. Ecology 101(4):e02978
Bhattacharyya A, Shah SK, Chaudhary V (2006) Would tree-ring data of Betula utilis have potential for the analysis of Himalayan glacial fluctuations. Curr Sci 91:754–761
Bobrowski M, Gerlitz L, Schickhoff U (2017) Modelling the potential distribution of Betula utilis in the Himalaya. Global Ecol Conserv 11:69–83
Bojarczuk K, Kieliszewska-Rokicka B (2010) Effect of ectomycorrhiza on Cu and Pb accumulation in leaves and roots of silver birch (Betula pendula Roth.) seedlings grown in metal-contaminated soil. Water Air Soil Pollut 207:227–240
Bonfante P, Venice F (2020) Mucoromycota: going to the roots of plant-interacting fungi. Fungal Biol Rev 34(2):100–113
Botha A (2011) The importance and ecology of yeasts in soil. Soil Biol Biochem 43(1):1–8
Bruns TD, Taylor JW (2016) Comment on “Global assessment of arbuscular mycorrhizal fungus diversity reveals very low endemism.” Science 351(6275):826–826
Buckeridge KM, Grogan P (2008) Deepened snow alters soil microbial nutrient limitations in arctic birch hummock tundra. Appl Soil Ecol 39(2):210–222
Buee M, Reich M, Murat C, Morin E, Nilsson RH, Uroz S, Martin F (2009) 454 Pyrosequencing analyses of forest soils reveal an unexpectedly high fungal diversity. New Phytol 184(2):449–456
Chen YL, Xu TL, Veresoglou SD, Hu HW, Hao ZP, Hu YJ, Chen BD (2017) Plant diversity represents the prevalent determinant of soil fungal community structure across temperate grasslands in northern China. Soil Biol Biochem 110:12–21
Cieraad E (2012) Temperate oceanic treelines-Low temperature effects on photosynthesis and growth (Doctoral dissertation Durham University)
Clemmensen KE, Bahr A, Ovaskainen O, Dahlberg A, Ekblad A, Wallander H, Stenlidr J, Finlayd DA, Lindahl BD (2013) Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science 339(6127):1615–1618
Clemmensen KE, Durling MB, Michelsen A, Hallin S, Finlay RD, Lindahl BD (2021) A tipping point in carbon storage when forest expands into tundra is related to mycorrhizal recycling of nitrogen. Ecol Lett 24(6):1193–1204
Cloete KJ, Valentine AJ, Stander MA, Blomerus LM, Botha A (2009) Evidence of symbiosis between the soil yeast Cryptococcus laurentii and a sclerophyllous medicinal shrub Agathosma betulina (Berg.) Pillans. Microb Ecol 57(4):624–632
Cripps CL, Eddington LH (2005) Distribution of mycorrhizal types among alpine vascular plant families on the Beartooth Plateau Rocky Mountains USA in reference to large-scale patterns in arctic–alpine habitats. Arct Antarct Alp Res 37(2):177–188
Cui L, Mu LQ (2016) Ectomycorrhizal communities associated with Tilia amurensis trees in natural versus urban forests of Heilongjiang in northeast China. J for Res 27(2):401–406
Dahlberg A, Bültmann H, Cripps CL, Eyjylfsdyttir G, Gulden G, Kristinsson H, Zhurbenko M (2013) Chapter 10. Arctic Biodiversity Assessment. Status and Trends in Arctic Biodiversity. Conservation of Arctic Flora and Fauna (CAFF). Narayana Press, Denmark.
Dar GH, Ganai NA, Beigh MA, Ahanger FA, Sofi TA (2010) Biodiversity of macro-fungi from conifer dominated forests of Kashmir, India. J Mycol Plant Pathol 40:169–171
Dasila K, Pandey A, Samant SS, Pande V (2020) Endophytes associated with Himalayan silver birch (Betula utilis D.Don) roots in relation to season and soil parameters. Appl Soil Ecol 149:103513
de Román M, de Miguel AM (2005) Post-fire, seasonal and annual dynamics of the ectomycorrhizal community in a Quercus ilex L. forest over a 3-year period. Mycorrhiza 15:471–482
Deslippe JR, Simard SW (2011) Below-ground carbon transfer among Betula nana may increase with warming in Arctic tundra. New Phytol 192(3):689–698
Ding Q, Liang Y, Legendre P, He X-H, Pei K-Q, Du X-J, Ma K-P (2011) Diversity and composition of ectomycorrhizal community on seedling roots: the role of host preference and soil origin. Mycorrhiza 21:669–680
Du C, Geng Z, Wang Q, Zhang T, He W, Hou L, Wang Y (2017) Variations in bacterial and fungal communities through soil depth profiles in a Betula albosinensis forest. J Microbiol 55(9):684–693
Eroshin VK, Dedyukhina EG (2002) Effect of lipids from Mortierella hygrophila on plant resistance to phytopathogens World. J Microbiol Biotechnol 18(2):165–167
Fajardo A, Piper FI, Cavieres LA (2011) Distinguishing local from global climate influences in the variation of carbon status with altitude in a tree line species. Glob Ecol Biogeogr 20(2):307–318
Fell JW, Statzell-Tallman A, Scorzetti G, Gutiérrez MH (2011) Five new species of yeasts from fresh water and marine habitats in the Florida Everglades. Antonie Van Leeuwenhoek 99(3):533–549
Gaire NP, Bhuju DR, Koirala M (2013) Dendrochronological studies in Nepal: current status and future prospects. FUUAST J Biol 3:1–9
Gao Q, Yang ZL (2016) Diversity and distribution patterns of root-associated fungi on herbaceous plants in alpine meadows of southwestern China. Mycologia 108(2):281–291
Geml J, Morgado LN, Semenova TA, Welker JM, Walker MD, Smets E (2015) Long-term warming alters richness and composition of taxonomic and functional groups of arctic fungi. FEMS Microbiol Ecol 91(8)
Gibson F, Fox FM, Deacon JW (1988) Effects of microwave treatment of soil on growth of birch (Betula pendula) seedlings and infection of them by ectomycorrhizal fungi. New Phytol 108(2):189–204
Goldmann K, Schöning I, Buscot F, Wubet T (2015) Forest management type influences diversity and community composition of soil fungi across temperate forest ecosystems. Front Microbiol 6:1300
Hagler AN, Mendonça-Hagler LC, Pagnocca FC (2017) Yeasts in aquatic ecotone habitats in Yeasts in natural ecosystems. Diversity. Springer, Cham, pp 63–85
Hamid M, Khuroo AA, Charles B, Ahmad R, Singh CP, Aravind NA (2019) Impact of climate change on the distribution range and niche dynamics of Himalayan birch a typical treeline species in Himalayas. Biodivers Conserv 28(8):2345–2370
Han Q, Huang J, Long D, Wang X, Liu J (2017) Diversity and community structure of ectomycorrhizal fungi associated with Larix chinensis across the alpine treeline ecotone of Taibai Mountain. Mycorrhiza 27(5):487–497
Hannula SE, Träger S (2020) Soil fungal guilds as important drivers of the plant richness–productivity relationship. New Phytol 226(4):947
Hassan MK, McInroy JA, Kloepper JW (2019) The interactions of rhizodeposits with plant growth-promoting rhizobacteria in the rhizosphere: a review. Agriculture 9(7):142
Hobbie JE, Hobbie EA (2006) 15N in symbiotic fungi and plants estimates nitrogen and carbon flux rates in Arctic tundra. Ecology 87(4):816–822
Hobbie EA, Ouimette AP (2009) Controls of nitrogen isotope patterns in soil profiles. Biogeochemistry 95(2):355–371
Högberg MN, Högberg P (2002) Extramatrical ectomycorrhizal mycelium contributes one-third of microbial biomass and produces together with associated roots half the dissolved organic carbon in a forest soil. New Phytol 154(3):791–795
Hrynkiewicz K, Baum C, Leinweber P (2009) Mycorrhizal community structure microbial biomass P and phosphatase activities under Salix polaris as influenced by nutrient availability. Eur J Soil Biol 45(2):168–175
Ishida TA, Nara K, Hogetsu T (2007) Host effects on ectomycorrhizal fungal communities: insight from eight host species in mixed conifer–broadleaf forests. New Phytol 174(2):430–440
Jumpponen A, Jones KL (2009) Massively parallel 454 sequencing indicates hyperdiverse fungal communities in temperate Quercus macrocarpa phyllosphere. New Phytol 184(2):438–448
Kalliokoski T (2011) Root system traits of Norway spruce, Scots pine, and silver birch in mixed boreal forests: an analysis of root architecture, morphology, and anatomy. Dissertation, Dissertationes Forestales 121. Vantaa, p 67
Körner C (1998) A re-assessment of high elevation treeline positions and their explanation. Oecologia 115(4):445–459
Körner C, Paulsen J (2004) A world-wide study of high altitude treeline temperatures. J Biogeogr 31(5):713–732
Körner C (2012) Water nutrient and carbon relations In Alpine Treelines. Springer, Basel, 151–168
Liang E, Dawadi B, Pederson N, Eckstein D (2014) Is the growth of birch at the upper timberline in the Himalayas limited by moisture or by temperature? Ecology 95(9):2453–2465
Männistö M, Vuosku J, Stark S, Saravesi K, Suokas M, Markkola A, Martz F, Rautio P (2018) Bacterial and fungal communities in boreal forest soil are insensitive to changes in snow cover conditions. FEMS Microbiol Ecol 94:fiy123
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular—arbuscular mycorrhizal fungi. New Phytol 115(3):495–501
Morgado LN, Semenova TA, Welker JM, Walker MD, Smets E, Geml J (2015) Summer temperature increase has distinct effects on the ectomycorrhizal fungal communities of moist tussock and dry tundra in Arctic Alaska. Glob Change Biol 21(2):959–972
Mühlmann O, Peintner U (2008) Mycobionts of Salix herbacea on a glacier forefront in the Austrian Alps. Mycorrhiza 18(4):171–180
Nara K (2006) Ectomycorrhizal networks and seedling establishment during early primary succession. New Phytol 169(1):169–178
Nguyen D, Boberg J, Cleary M, Bruelheide H, Hönig L, Koricheva J, Stenlid J (2017) Foliar fungi of Betula pendula: impact of tree species mixtures and assessment methods. Sci Rep 7(1):1–11
Northup RR, Yu Z, Dahlgren RA, Vogt KA (1995) Polyphenol control of nitrogen release from pine litter. Nature 377(6546):227–229
Peay KG, Baraloto C, Fine PV (2013) Strong coupling of plant and fungal community structure across western Amazonian rainforests. ISME J 7(9):1852–1861
Peterson RL, Bonfante P (1994) Comparative structure of vesicular arbuscular mycorrhizas and ectomycorrhizas. Plant Soil 159:79–88
Preusser S, Poll C, Marhan S, Angst G, Mueller CW, Bachmann J, Kandeler E (2019) Fungi and bacteria respond differently to changing environmental conditions within a soil profile. Soil Biol Biochem 137:107543
Qin GZ, Tian SP (2004) Biocontrol of postharvest diseases of jujube fruit by Cryptococcus laurentii combined with a low dosage of fungicides under different storage conditions. Plant Dis 88(5):497–501
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Rana KL, Kour D, Sheikh I, Yadav N, Yadav AN, Kumar V, Singh BP, Dhaliwal HS, Saxena AK (2019) Biodiversity of endophytic fungi from diverse niches and their biotechnological applications. Adv Endophytic Fungal Res 105–144
Safdar I, Bibi Y, Hussain M, Iqbal M, Saira H, Shaheen S, Mehboob H (2017) Review on current status of Betula utilis: An important medicinal plant from Himalaya. J Bot Sci 6:1–7
Sakai A, Larcher W (1987) Low temperature and frost as environmental factors. Frost Survival of Plants. Springer, Berlin, Heidelberg, pp 1–20
Semenova TA, Morgado LN, Welker JM, Walker MD, Smets E, Geml J (2015) Long-term experimental warming alters community composition of ascomycetes in Alaskan moist and dry arctic tundra. Mol Ecol 24(2):424–437
Shi S, Nuccio EE, Shi ZJ, He Z, Zhou J, Firestone MK (2016) The interconnected rhizosphere: high network complexity dominates rhizosphere assemblages. Ecol Lett 19(8):926–936
Shrestha BB, Ghimire B, Lekhak HD, Jha PK (2007) Regeneration of treeline birch (Betula utilis D. Don) forest in a trans-Himalayan dry valley in central Nepal. Mt Res Dev 27(3):259–267
Smith SE, Read DJ (2010) Mycorrhizal symbiosis. Academic press.
Soudzilovskaia NA, Douma JC, Akhmetzhanova AA, van Bodegom PM, Cornwell WK, Moens EJ, Kathleen K, Treseder MT, Ying PW, Cornelissen JH (2015) Global patterns of plant root colonization intensity by mycorrhizal fungi explained by climate and soil chemistry. Glob Ecol Biogeogr 24(3):371–382
Speed JD, Austrheim G, Hester AJ, Mysterud A (2011) Growth limitation of mountain birch caused by sheep browsing at the altitudinal treeline. For Ecol Manag 261(7):1344–1352
Splivallo R, Fischer U, Gobel C, Feussner I, Karlovsky P (2009) Truffles regulate plant root morphogenesis via the production of auxin and ethylene. Plant Physiol 150(4):2018–2029
Steidinger BS, Crowther TW, Liang J, Van Nuland ME, Werner GD, Reich PB, Peay KG (2019) Climatic controls of decomposition drive the global biogeography of forest-tree symbioses. Nature 569(7756):404–408
Stevens GC, Fox JF (1991) The causes of treeline. Annu Rev Ecol Syst 22(1):177–191
Talbot JM, Martin F, Kohler A, Henrissat B, Peay KG (2015) Functional guild classification predicts the enzymatic role of fungi in litter and soil biogeochemistry. Soil Biol Biochem 88:441–456
Tarkka MT, Herrmann S, Wubet T, Feldhahn L, Recht S, Kurth F, Buscot F (2013) OakContig DF 159.1 a reference library for studying differential gene expression in Quercus robur during controlled biotic interactions: use for quantitative transcriptomic profiling of oak roots in ectomycorrhizal symbiosis. New Phytol 199(2):529–540
Tedersoo L, Smith ME (2013) Lineages of ectomycorrhizal fungi revisited: foraging strategies and novel lineages revealed by sequences from belowground. Fungal Biol Rev 27(3–4):83–99
Tedersoo L, Jairus T, Horton BM, Abarenkov K, Suvi T, Saar I, Kõljalg U (2008) Strong host preference of ectomycorrhizal fungi in a Tasmanian wet sclerophyll forest as revealed by DNA barcoding and taxon-specific primers. New Phytol 180(2):479–490
Tedersoo L, Nilsson RH, Abarenkov K, Jairus T, Sadam A, Saar I, Bahram M, Bechem E, Chuyong G, Kõljalg U (2010) 454 Pyrosequencing and Sanger sequencing of tropical mycorrhizal fungi provide similar results but reveal substantial methodological biases. New Phytol 188:291–301
Tedersoo L, Bahram M, Cajthaml T, Põlme S, Hiiesalu I, Anslan S, Abarenkov K (2016) Tree diversity and species identity effects on soil fungi protists and animals are context dependent. ISME J 10(2):346–362
Tewari A, Shah S, Singh N, Mittal A (2018) Treeline species in Western Himalaya are not water stressed: a comparison with low elevation species. Trop Ecol 59(2)
Tibbett M, Sanders FE (2002) Ectomycorrhizal symbiosis can enhance plant nutrition through improved access to discrete organic nutrient patches of high resource quality. Ann Bot 89(6):783–789
Truong C, Palmé AE, Felber F (2007) Recent invasion of the mountain birch Betula pubescens ssp tortuosa above the treeline due to climate change: genetic and ecological study in northern Sweden. J Evol Biol 20(1):369–380
Tyub S, Kamili AN, Reshi ZA, Rashid I, Mokhdomi TA, Bukhari S, Amin A, Wafai AH, Qadri RA (2018) Root-associated fungi of Pinus wallichiana in Kashmir Himalaya. Can J For Res 48(8):923–929
Van Der Putten WH (2017) Belowground drivers of plant diversity. Science 355(6321):134–135
Wani MS, Gupta RC, Munshi AH, Sharma V (2018) Genetic diversity and structure of Betula utilis accessions of North-western Himalaya based on RAPD and ISSR markers. Nucleus 61(2):145–152
Wardle P (2008) New Zealand Forest to alpine transitions in global context. Arct Antarct Alp Res 40(1):240–249
Wardle D (1993) Changes in the microbial biomass and metabolic quotient during leaf litter succession in some New Zealand forest and scrubland ecosystems. Funct Ecol 346–355
Xu Z, Hu T, Zhang Y (2012) Effects of experimental warming on phenology growth and gas exchange of treeline birch (Betula utilis) saplings Eastern Tibetan Plateau China. Eur J For Res 131(3):811–819
Yang L, Xie J, Jiang D, Fu Y, Li G, Lin F (2008) Antifungal substances produced by Penicillium oxalicum strain PY-1—potential antibiotics against plant pathogenic fungi. World J Microbiol Biotechnol 24(7):909–915
Yang MZ, Ma MD, Yuan MQ, Huang ZY, Yang WX, Zhang HB, Huang LH, Ren AY, Shan H (2016) Fungal endophytes as a metabolic fine-tuning regulator for wine grape. PLoS ONE 11(9):e0163186
Acknowledgements
Our explicit acknowledgement goes to the YAAZ xenomics Coimbatore, Tamil Nadu India, for supporting and providing lab to carry out Next Generation Amplicon Sequencing related work. We also thank support staff of GAIA pipeline Barcelona, Spain for helping in data analysis.
Funding
The study was partially supported under the Timberline project supported by the Ministry of Environment, Forest and Climate change (New Delhi) thorough G.B. Pant National Institute of Himalayan Environment and Sustainable Development (Almora), Uttarakhand grant to University of Kashmir, Srinagar.
Author information
Authors and Affiliations
Contributions
Conceived study and sampling design of experiment: ZAR and NFK. Conducted fieldwork, processed and analyzed samples: NFK. Analyzed data and submitted data: NFK. Wrote and refined paper: ZAR and NFK.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
The original online version of this article was revised: Modifications have been made in the text, to several section headings and also to Table 1. Full information regarding the corrections made can be found in the erratum/correction for this article.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khan, N.F., Reshi, Z.A. Diversity of root-associated mycobiome of Betula utilis D. Don: a treeline species in Kashmir Himalaya. Trop Ecol 63, 531–546 (2022). https://doi.org/10.1007/s42965-022-00230-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42965-022-00230-4