Abstract
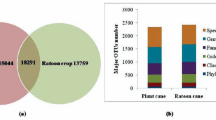
India is a major producer and consumer of seed spices. There are numbers of studies have been done on soil and crop parameters of these but there is lacuna on the study of soil bacterial community analysis in the seed spices. Soil microorganisms are known to play critical role in soil biochemical processes, nutrient cycling, soil fertility, and also influences plant performances. With the objectives to know microbial community composition and soil chemical and biological properties in rhizosphere of two seed spices plants- cumin and coriander we collected five soil samples from rhizosphere of cumin (NRCSS-SL-1 and NRCSS-SL-3) and coriander (NRCSS-SL-2, NRCSS-SL-4 and NRCSS-SL-5) grown in arid and semi-arid regions of India. Electrical conductivity (0.32–0.53 ds/m), organic carbon (0.03–0.64%), total nitrogen (223–258 kg/ha), total phosphorous (30–47.3 kg/ha) and total potash (206–388 kg/ha) were varied in samples. In biological properties bacterial and fungal populations were varied between 98.33 and 210 c.f.u. × 104 g−1 soil and 32–69.33 c.f.u. × 103 g−1 soil, respectively. Different soil enzyme activities dehydrogenase (37.96–57.68 µg TPF g−1 soil 24 h−1), acid phosphatase (14.66–24.72 µg p-NP g−1 soil h−1), and alkaline phosphatase (27.08–37.58 µg p-NP g−1 soil h−1) were varied in samples. Soil chemical and biological activities were found highest in NRCSS-SL-2 followed by NRCSS-SL-5 and lowest in NRCSS-SL-1. Amplicon sequencing of 16S rRNA gene was done in the collected samples to identify and quantify the bacterial community structure. A total 37 phyla were observed with varying abundances in different samples. Next generation sequencing shows that three phyla were present in order of Firmicutes (26–43%) > Proteobacteria (23–29%) > Actinobacteria (11–22%) and they were dominating in all the soil samples. Relative abundance of order Bacillales was highest (26–35%), followed by Rhizobiales (8–10%) and Actinomycetales (7–11%) in samples. There were many orders which found in very low abundance and moreover some were not present in all soil samples but these may play significant role in the soil ecosystem. The study concludes that rhizosphere soil of seed spices harbors wide range of unculturable microbes and climatic conditions have impact on their community structure. Microbial activities in terms of microbial colony count and enzyme activity are also influenced by soil chemical properties.



Similar content being viewed by others
Data availability
The metagenome dataset with information about taxonomic and other details is publicly available on National Centre for Biotechnology Information Sequence Read Archive under bioproject PRJNA377848 having five biosamples with accession numbers SAMN06473293 (NRCSS-SL-1), SAMN06473335 (NRCSS-SL-2), SAMN06473337 (NRCSS-SL-3), SAMN06473339 (NRCSS-SL-4) and SAMN06473340 (NRCSS-SL-5).
References
Bandick AK, Dick RP (1999) Field management effects on soil enzyme activities. Soil Biol Biochem 31(11):1471–1479
Bargaz A, Lyamlouli K, Chtouki M, Zeroul Y, Dhiba D (2018) Soil microbial resources for improving fertilizers efficiency in an integrated plant nutrient management system. Front Microbiol 9:1606
Bolton Jr H, Elliott LF, Papendick RI, Bezdicek DF (1985) Soil microbial biomass and selected soil enzyme activities: effect of fertilization and cropping practices. Soil Biol Biochem 17(3):297–302
Breulmann M, Masyutenko NP, Kogut BM, Schroll R, Dörfler U, Buscot F, Schulz E (2014) Short-term bioavailability of carbon in soil organic matter fractions of different particle sizes and densities in grassland ecosystems. Sci Total Environ 497:29–37
Casida LE Jr, Klein DA, Santoro T (1964) Soil dehydrogenase activity. Soil Sci 98(6):371–376
Chaudhary GR (2013) Advances in production technologies for major seed spices for higher productivity. Resource conservation technologies of seed spices 137–144
Choudhary M, Datta A, Jat HS, Yadav AK, Gathala MK, Sapkota TB, Das AK, Sharma PC, Jat ML, Singh R, Ladha JK (2018a) Changes in soil biology under conservation agriculture based sustainable intensification of cereal systems in Indo-Gangetic Plains. Geoderma 313:193–204
Choudhary M, Sharma PC, Jat HS, Dash A, Rajashekar B, McDonald AJ, Jat ML (2018b) Soil bacterial diversity under conservation agriculture-based cereal systems in Indo-Gangetic Plains. 3 Biotech 8(7):304
Choudhary M, Jat HS, Datta A, Sharma PC, Rajashekar B, Jat ML (2020) Topsoil bacterial community changes and nutrient dynamics under cereal based climate-smart agri-food systems. Front Microbiol 11:1812
Compant S, Samad A, Faist H, Sessitsch A (2019) A review on the plant microbiome: ecology, functions and emerging trends in microbial application. J Adv Res 19:29–37
Cui Y, Fang L, Guo X, Wang X, Wang Y, Zhang Y, Zhang X (2019) Responses of soil bacterial communities, enzyme activities, and nutrients to agricultural-to-natural ecosystem conversion in the Loess Plateau. China J Soils Sediments 19(3):1427–1440
Curtis TP, Sloan WT, Scannell JW (2002) Estimating prokaryotic diversity and its limits. Proc Nat Acad Sci 99(16):10494–10499
Darrah PR (1991) Models of the rhizosphere. Plant Soil 133(2):187–199
Das SK, Varma A (2010) Role of enzymes in maintaining soil health. In Soil enzymology. Springer, Berlin, pp 25–42
Deng J, Zhang Y, Yin Y, Zhu X, Zhu W, Zhou Y (2019) Comparison of soil bacterial community and functional characteristics following afforestation in the semi-arid areas. PeerJ 7:e7141
de la Porte A, Schmidt R, Yergeau É, Constant P (2020) A gaseous milieu: extending the boundaries of the rhizosphere. Trend Microbiol 28(7):536–542
Eilers KG, Lauber CL, Knight R, Fierer N (2010) Shifts in bacterial community structure associated with inputs of low molecular weight carbon compounds to soil. Soil Biol Biochem 42:896–903
Fanin N, Kardol P, Farrell M, Nilsson MC, Gundale MJ, Wardle DA (2019) The ratio of Gram-positive to Gram-negative bacterial PLFA markers as an indicator of carbon availability in organic soils. Soil Biol Biochem 128:111–114
Fierer N, Bradford M, Jackson R (2007) Toward an ecological classification of soil bacteria. Ecology 88:1354–1364
Fonseca-García C, Coleman-Derr D, Garrido E, Visel A, Tringe SG, Partida-Martínez LP (2016) The cacti microbiome: interplay between habitat-filtering and host-specificity. Front Microbiol 7:150
Hartmann A, Schmid M, Van Tuinen D, Berg G (2009) Plant-driven selection of microbes. Plant Soil 321(1–2):235–257
Ho A, Di Lonardo DP, Bodelier PL (2017) Revisiting life strategy concepts in environmental microbial ecology. FEMS Microbiol Ecol 93(3):006
Hol WH, De Boer W, de Hollander M, Kuramae EE, Meisner A, Van Der Putten WH (2015) Context dependency and saturating effects of loss of rare soil microbes on plant productivity. Front Plant Sci 6:485
Jacoby R, Peukert M, Succurro A, Koprivova A, Kopriva S (2017) The role of soil microorganism in plant mineral nutrition-current knowledge and future directions. Front Plant Sci 8:1617
Jain BL, Singh RS, Shyampura RL, Maji AK (2007) Soil site suitability for major crops in arid and semi arid region. NBSS Publication, NBSS&LUP, Nagpur
Jat HS, Choudhary M, Datta A, Yadav AK, Meena MD, Devi R, Gathala MK, Jat ML, McDonald A, Sharma PC (2020). Temporal changes in soil microbial properties and nutrient dynamics under climate smart agriculture practices. Soil and Tillage Research 199:104595
Jackson ML (1973) Soil chemical analysis. Prentice Hall of India Pvt. Ltd., New Delhi
Jousset A, Bienhold C, Chatzinotas A, Gallien L, Gobet A, Kurm V, Küsel K, Rillig MC, Rivett DW, Salles JF, Van Der Heijden MG (2017) Where less may be more: how the rare biosphere pulls ecosystems strings. ISME J 11(4):853–862
Karthikeyan AS, Varadarajan DK, Mukatira UT, D’Urzo MP, Damaz B, Raghothama KG (2002) Regulated expression of arabidopsis phosphate transporters. Plant Physiol 130:221–233
Kurm V, Geisen S, Gera Hol WH (2019) A low proportion of rare bacterial taxa responds to abiotic changes compared with dominant taxa. Environ Microbiol 21(2):750–758
Lambrechts S, Willems A, Tahon G (2019) Uncovering the uncultivated majority in Antarctic soil: towards a synergistic approach. Front Microbiol 10:3389
Locey KJ, Lennon JT (2016) Scaling laws predict global microbial diversity. Proc Natl Acad Sci USA 113:5970–5975
Luo L, Meng H, Gu JD (2017) Microbial extracellular enzymes in biogeochemical cycling of ecosystems. J Environ Manage 197:539–549
Mandic-Mulec I, Prosser JI (2011) Diversity of endospore-forming bacteria in soil: characterization and driving mechanisms. In: Endospore-forming soil bacteria. Springer, Berlin, pp 31–59
Martins LF, Antunes LP, Pascon RC, de Oliveira JCF, Digiampietri LA, Barbosa D, Peixoto BM, Vallim MA, Viana-Niero C, Ostroski EH, Telles GP (2013) Metagenomic analysis of a tropical composting operation at the São Paulo Zoo Park reveals diversity of biomass degradation functions and organisms. PloS one 8(4):61928
Mayer Z, Zita S, Viktor S, Beatrix PR, Balázs V, Katalin P (2019) Effect of long-term cropping systems on the diversity of the soil bacterial communities. Agronomy 9(12):878
Mendes LW, Kuramae EE, Navarrete AA, Van Veen JA, Tsai SM (2014) Taxonomical and functional microbial community selection in soybean rhizosphere. ISME J 8(8):1577–1587
Mishra BK, Meena KK, Dubey PN, Aishwath OP, Krishna K, Sorty Ajay M, Bitla U (2016) Influence on yield and quality of fennel (Foeniculum vulgare Mill.) grown under semi-arid saline soil, due to application of native phosphate solubilizing rhizobacterial isolates. Ecol Eng 97:327–333
Morita RY (1988) Bioavailability of energy and its relationship to growth and starvation survival in nature. Can J Microbiol 34(4):36–441
Nehra V, Choudhary M (2015) A review on plant growth promoting rhizobacteria acting as bioinoculants and their biological approach towards the production of sustainable agriculture. J Appl Natl Sci 7(1):540–556
Olsen SR, Cole CV, Watenale FS, Dean LA (1954) Estimation of available phosphorus in soil by extraction with sodium bicarbonate. USDA Circ 939, Washington, D.C
Prarg N, Pauli H, Paul I (2019) Microbial diversity in bulk and rhizosphere soil of Ranunculus gracialis along a high-alpine altitudinal gradient. Front Microbiol 10:1429
Price MN, Dehal PS, Arkin AP (2010) FastTree 2–approximately maximum-likelihood trees for large alignments. PloS one 5(3):e9490
Shakya M, Gottel N, Castro H, Yang ZK, Gunter L, Labbé J (2013) A multifactor analysis of fungal and bacterial community structure in the root microbiome of mature Populus deltoides trees. PLoS ONE 8:e76382
Philippot L, Raaijmakers JM, Lemanceau P, Van Der Putten WH (2013) Going back to the roots: the microbial ecology of the rhizosphere. Nat Rev Microbiol 11(11):789–799
Sharma SS, Rao SS, Singh RS, Sharma RP, Dubey PN (2018) Evaluation of potential cumin growing area in hot arid region of Jaisalmer district. Int J Seed Spices 8(1):50–55
SPSS Inc Released (1999) SPSS for Windows, Version 17.0. Chicago
Stewart E (2012) Growing unculturable bacteria. J Bacteriol 194:415160
Subbiah B, Asija GL (1956) Alkaline permanganate method of available nitrogen determination. Curr Sci 25:259
Tabatabai MA (1994) Soil enzymes. Methods of soil analysis. Part 2. Microbiol Biochem Properties 5:775–833
Tapsell LC, Hemphill I, Cobiac L, Patch CS, Sullivan DR, Fenech M, Roodenrys S, Keogh JB, Clifton PM, Williams PG, Fazio VA, Inge KE (2006) Health benefits of herbs and spices: the past, the present, the future. Med J 185(S4 I):S1–S24
Timmusk S, Behers L, Muthoni J, Muraya A, Aronsson AC (2017) Perspectives and challenges of microbial application for crop improvement. Front Plant Sci 8
Toju H, Peay KG, Yamamichi M, Narisawa K, Hiruma K, Naito K, Fukuda S, Ushio M, Nakaoka S, Onoda Y, Yoshida K (2018) Core microbiomes for sustainable agroecosystems. Nature Plants 4(5):247–257
Torsvik V, Ovreas L (2002) Microbial diversity and function in soil: from genes to ecosystem. Curr Opin Microbiol 5:240–245
UN Food & Agriculture Organization (2011) Production of Spice by countries. Archived from the original on July 13, 2011. Retrieved December 20, 2013
Utobo EB, Tewari L (2015) Soil enzymes as bioindicators of soil ecosystem status. Appl Ecol Environ Res 13(1):147–169
Vančura V, Hovadík A (1965) Root exudates of plants. Plant Soil 22:21–32. https://doi.org/10.1007/BF01377686
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci 37(1):29–38
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73(16):5261–5267
Wohlgemuth S, Kämpfer P (2014) BACTERIA| Bacterial Endospores. Encyclopedia of Food Microbiology (Second Edition): 160–168
Acknowledgements
We thank Director, NRCSS for timely support and encouragement during the research work. The research is supported by the ICAR under AMAAS project scheme.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
We have no conflicts of interest.
Ethical approval
Our research does not include Human Participants and/or Animals.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Choudhary, S., Mishra, B.K., Singh, R. et al. Bacterial diversity and bio-chemical properties in the rhizosphere soils of Cumin and Coriander. Trop Ecol 62, 368–376 (2021). https://doi.org/10.1007/s42965-021-00155-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42965-021-00155-4