Abstract
In tropical ecosystems, interactions between arbuscular mycorrhizal fungi (AMF) and other organisms have been little studied, but may be of significant importance for understanding the role of AMF in decomposition processes and nutrient cycling. In this study, we used ingrowth cores to investigate the impacts of regular rotation of the cores, defaunation and nitrogen addition on AMF, microbial biomass and microarthropods in the fermentation/humus (F/H) and litter (L) layers of an Ecuadorian montane tropical rainforest. AMF were substantially reduced in the F/H layer (to 34% of initial), while in the L layer they remained constant during the experiment. Overall, microorganisms and microarthropods were largely independent of AMF hyphae and their exudates, however, defaunation strongly affected the recovery of their communities. Nitrogen addition increased the quality of litter material and beneficially affected microbial communities thereby increasing decomposition rates, but did not impact AMF abundance and microarthropod communities. These findings suggest that the cutoff of the carbon supply from the plant to the fungal mycelium was not compensated by switching resources in the F/H layer, underlining a strong association between AMF and living roots. While in the L layer, AMF likely competed with saprotrophic microorganisms for litter-derived resources at intermediate stages of decomposition pointing to indirect contributions of AMF to decomposition processes. Overall, the results support the view that root-derived resources are important in fueling soil food webs, but also indicate that in the studied montane rainforest these resources are only available close to roots and not channeled distant to roots via AMF.
Similar content being viewed by others
Introduction
Mycorrhizal fungi are key components of microorganisms in soil influencing plant nutrient uptake and growth (Johansson et al. 2004; Brundrett and Tedersoo 2018). In tropical forest ecosystems, arbuscular mycorrhiza fungi (AMF; Glomeromycotina) represent the dominant mycorrhizal form (Kottke et al. 2004; Öpik et al. 2006). These obligate biotrophs facilitate the mobilization and uptake of mineral by plants released from decomposition of organic matter in exchange for photosynthetic carbon (Read and Perez-Moreno 2003; Smith and Read 2008; Johnson 2010; Bagyaraj 2014). Factors that affect the abundance and effectiveness of AMF include climatic changes, soil fertility, disturbances and changes in nutrient availability (Gryndler 2000; Cardoso and Kuyper 2006; Camenzind et al. 2014; Lehmann et al. 2017). Elevated nutrient availability—mainly P and N—decreases the formation of fine roots and mycorrhizal structures, reducing the benefit provided by these symbionts (Treseder and Allen 2002; Johnson 2010). Therefore, changes following nutrient additions might strongly affect tropical forests where P and N are limiting and plants rely on AMF symbionts (Cardoso and Kuyper 2006; Dalling et al. 2016; Sheldrake et al. 2018).
In addition to roots, AMF interact with other organisms including bacteria, saprotrophic fungi, protozoa, nematodes, arthropods and even large animals (Ruess and Lussenhop 2005; Miransari 2011; Lehmann et al. 2017). These interactions may be synergistic, competitive or antagonistic and may affect all stages of the mycorrhizal fungal life cycle (Finlay 2004; Johansson et al. 2004; Miransari 2011). Interacting mechanisms which are of particular importance for the functioning of the symbiosis include mycorrhiza-associated bacteria promoting or inhibiting mycorrhiza formation (Toljander et al. 2007; Svenningsen et al. 2018), potential restriction of saprotrophic fungal activity due to competition for substrate (Bödeker et al. 2016; Marian et al. 2019), and grazing of external mycelium by soil microarthropods (Ruess and Lussenhop 2005; A’Bear et al. 2014). The most abundant and frequent groups of soil microarthropods are mites (Acari) and springtails (Collembola) (Maraun et al. 2003; Franklin et al. 2004). Particularly oribatid mites (Oribatida) are rich in species and colonize virtually any soil reaching maximum diversity and density in forest ecosystems, where they participate in the decomposition of organic matter (Franklin et al. 2004). Oribatid mites feed on a variety of fungal species and contribute to the dispersion of fungal structures (Renker et al. 2005; Vašutová et al. 2019). However, AMF presumably are not the preferred food resource of oribatid mites, but might be consumed to some extent (Gange and Brown 2002; Schneider et al. 2005).
Typically, interactions between soil microarthropods and AMF are studied using sterilized soil re-inoculated with microorganisms and microarthropods (Toljander et al. 2007; Nuccio et al. 2013; Ngosong et al. 2014). For quantifying AMF biomass, as well as their contribution to nutrient translocation, plant growth and their interactions with microbial communities, ingrowth cores are increasingly used (Nottingham et al. 2013; Leifheit et al. 2014). Ingrowth cores comprise compartments separated by mesh barriers for excluding roots, but allowing access of extraradical mycorrhizal hyphae, with soil physical and chemical conditions inside the cores resembling those outside the cores (Wallander et al. 2013). Rotating these cores detaches fungal ingrowth and thereby, the comparison of rotated and non-rotated cores provides insight into the role of mycorrhizal fungi for element cycling and decomposition (Johnson et al. 2001).
To explore the role of AMF in soil nutrient dynamics and their interactions with soil organisms in tropical montane rainforests, we evaluated the impact of rotation of ingrowth cores in combination with soil defaunation and N addition on AMF abundance, microorganisms and soil microarthropods, with focus on oribatid mites. We hypothesized that (1) the colonization of soil inside the cores by AMF is reduced by regular rotation. Assuming that mycorrhiza indirectly alter decomposition processes by restraining the activity of saprotrophic fungi, we furthermore hypothesized that (2) the exclusion of mycorrhiza results in increased activity of saprotrophic microorganisms, accelerating decomposition processes. Further, we hypothesized that (3) oribatid mites benefit from the increased dominance of saprotrophic fungi in rotated cores. Also, we hypothesized that (4) the reduction of microarthropod abundance in defaunated cores promotes fast recovery of AMF mycelium and soil microorganisms. Finally, we hypothesized that (5) the addition of N reduces the concentration of AMF, but fosters the activity of saprotrophic microorganisms and thereby increases litter decomposition.
Materials and methods
Study site
The study site is located in Southern Ecuador within the Podocarpus National Park near the research station San Francisco at 2000 m a.s.l. (3°58′S, 79°04′W) (for location details see Richter et al. 2009). The climate is warm humid with an average annual temperature of 15.2 °C and an annual precipitation of approximately 2000 mm. Precipitation is high from April to September, and lower between October and March, but there is no pronounced dry season (Bendix et al. 2006). The soil is stagnic cambisol with a thick organic layer ranging between 8 and 40 cm (Wilcke et al. 2002; Wullaert et al. 2010). The area is characterized by high diversity of organisms and a particular high number of endemic vascular plant species, with Melastomataceae, Lauraceae and Rubiaceae being the dominant plant families (Bendix et al. 2006; Beck and Ritcher 2008; Homeier et al. 2010). Roots of trees in the forests are colonized predominantly by AMF and are characterized by high rates of AMF colonization suggesting a high contribution of AMF hyphae to soil fungal biomass (Kottke et al. 2004; Camenzind and Rillig 2013).
Ingrowth core
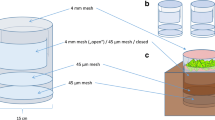
Ingrowth cores were constructed using 15 cm (diameter) × 20 cm (length) plastic tubes. Two rectangular holes (10 × 15 cm) opposite to each other were cut into the tubes and covered with 45 µm nylon mesh. Two layers of 45 µm nylon mesh, separated by 5 cm, were glued inside the tubes to allow drainage of leaching water but block ingrowth of roots and mycorrhizal hyphae from the bottom. The ingrowth cores were closed with a lid of 4 mm nylon mesh to allow mesofauna access (Fig. 1a).
Scheme of the ingrowth cores and representation of experimental design. a Scheme of the ingrowth cores, see text for details. b Representation of the experimental design per subplot with the ingrowth cores inserted in the soil and litter material placed on top of the soil inside the cores separated from the soil by 4 mm mesh; four ingrowth cores were placed per subplot; R–ND rotated and non-defaunated, R–D rotated and defaunated, NR–ND non-rotated and non-defaunated, NR–D non-rotated and defaunated; arrows indicate rotation by 45° every second day
Experimental design
The experiment started in June 2015 and was conducted in the framework of the Nutrient Manipulation Experiment (NUMEX) (Homeier et al. 2012). Briefly, NUMEX is an altitudinal fertilization experiment that was set up in a complete randomized block design with four blocks, each containing one plot (20 × 20 m) of four different treatments: addition of N (+ N), addition of P (+ P), addition of N and P (+ N + P) and unfertilized control plots (Ctr) (Wullaert et al. 2010; Homeier et al. 2012). For the present experiment, soil samples (~ 15 cm deep) were taken from two subplots (2 × 2 m) marked randomly inside the + N and Ctr plots of NUMEX at 2000 m with a stainless steel corer (14.5 cm inner diameter). The soil samples comprised the fermentation/humus (F/H) and litter (L) layers and were inserted intact into the ingrowth cores and placed into the same holes from which the soil sample were excavated. Prior to placement in the field, half of the prospective rotated and non-rotated cores were frozen at − 20 °C for 1 week to kill soil living animals. Once in the field, the soil sample was covered by 4 mm mesh on top of which 2 g of dry (60 °C, 48 h) Graffenrieda emarginata leaves, the most abundant tree species in the study area, were placed as standard litter material allowing to investigate the decomposition of leaf litter inside the cores (Fig. 1b). Decomposition of the added Graffenrieda litter was calculated as \({\text{mass }}\;{\text{loss}} \,\left( \% \right) = ((m_{0} - m_{1} /m_{0} )) \times 100,\) with m0 as the initial dry weight of the leaves and m1 as the dry weight of the leaves at harvest.
The cores were rotated every second day by 45° during 5 months. After removal of the cores by the end of the experiment, L and F/H layer materials were sampled separately. Half of each sample was used for the analysis of soil properties, the other half for extraction of microarthropods. Microarthropods were extracted by heat using a modified high gradient extractor (Macfadyen 1961; Kempson et al. 1963). Thereafter, they were determined to group level (Oribatida, Collembola, Prostigmata, Gamasina, Uropodina), with the exception of adult Oribatida which were identified to species level. For identification, the keys of Hammer (1958, 1961) and Balogh and Balogh (1990, 2002) were used and nomenclature followed Subías (2018).
Microbial respiration and microbial biomass
Microbial basal respiration (BR) and microbial biomass (Cmic) were determined by measuring O2 consumption using an automated respirometer system (Scheu 1992). The O2 consumption was measured every hour during 24 h at 22 °C. BR (μl O2 g−1 dry weight h−1) was calculated as mean of O2 consumption rates from 10 to 20 h after attachment of the samples to the respirometer system.
Cmic (μg g−1 dry weight) was determined by measuring the maximum initial respiratory response (MIRR; µl O2 g−1 h−1). Moist samples equivalent to 0.2 g dry weight were supplemented with d-glucose (80 mg g−1 and 40 mg g−1 dry weight for L and F/H layers, respectively). The average of the lowest three readings within the first 10 h was used as MIRR. Microbial biomass was calculated as Cmic = 38 × MIRR (SIR-method; Anderson and Domsch 1978; Beck et al. 1997).
Fatty acid analysis
Phospholipid fatty acids (PLFAs) and neutral lipid fatty acids (NFLAs) were extracted from L and F/H layer material, as well as from initial soil samples taken from each subplot following the protocol of Frostegård et al. (1993). Fatty acid methyl esters (FAMEs) were identified by chromatographic retention time based on a standard mixture composed of 37 different FAMEs ranging from C11 to C24 (Sigma-Aldrich, St Louis, USA). The analysis was performed by gas chromatography using a GC-FID Clarus 500 (PerkinElmer Corporation, Norwalk, USA) equipped with HP-5 capillary column (30 m × 0.32 mm id, film thickness 0.25 µm).
PLFAs and NLFAs were expressed in nmol g−1 dry weight. PLFAs were used as indicator of the microbial community structure. The sum of i15:0, a15:0, 15:0, i16:0, 16:1ω7, i17:0, 17:0, cy17:0, 18:1ω7 and cy19:0 was used as indicator of bacterial biomass (Frostegård et al. 1993; Frostegård and Bååth 1996). The sum of 16:1ω7, cy17:0, 18:1ω7 and cy19:0 was the indicator of Gram-negative and the sum of i15:0, a15:0, i16:0, i17:0 and a17:0 was the indicator of Gram-positive bacteria (Zelles 1997, 1999). The PLFAs 18:2ω6,9 and 18:1ω9 were used as markers for saprotrophic fungi (Frostegård and Bååth 1996; Ruess and Chamberlain 2010). The NLFA 16:1ω5 was used as marker for AMF (Olsson et al. 1995; Olsson 1999).
Carbon and nitrogen analyses
Soil pH was determined based on a 1:5 (v:v) suspension of soil in deionized water. Total C and N contents in soil and litter were determined from milled samples using an elemental analyzer (EuroEA, HekaTech, Germany). The fraction of Bray-extractable inorganic phosphorus (P) in soil was determined based on protocols described by Bray and Kurtz (1945). Available P was extracted from 2 g dry soil adding a solution containing hydrochloric acid (HCl) and ammonium fluoride (NH4F). The resulting P content in the solution filtered through phosphorus-free filter paper was analyzed by ICP-OES analyses (Optima 2100 DV, Perkin Elmer, Germany).
Statistical analyses
Prior to statistical analysis, the data were inspected for normality and homogeneity of variance using Shapiro–Wilk and Breusch–Pagan tests, respectively. If necessary, data were log- or arcsine-transformed to improve homogeneity of variances. Soil characteristics (concentration of C, N, PO43−, P and pH), microbial activity (BR and Cmic), fatty acids (NLFAs and PLFAs) and microarthropod groups (Oribatida, Gamasina, Uropodina, Astigmata and Prostigmata (all Acari) and Collembola) were analyzed using separated three-way linear mixed-effects models (LMM) with rotation, N addition and defaunation fitted as fixed effects and block fitted as random effect. Oribatid mite community data were compressed using non-metric multidimensional scaling (NMDS). Only species with more than three individuals in the samples were included. The stress value obtained reduced the number of meaningful dimensions to six axes. Afterwards, multivariate analysis of variance (MANOVA) was used to inspect effects of treatments on oribatid mite community composition. Additionally, using the same data set, principal components analysis (PCA) was used to analyze and present graphically the response of oribatid mite species to rotation, N addition and defaunation in the L and F/H layer. The soil layer (L and F/H layer) and the three treatments (Rotation, N addition and defaunation) were coded as supplementary variables not affecting the ordination. Only species that were present in more than three samples were included in the analysis. PCA was carried out using CANOCO 5 (Ter Braak and Smilauer 2012). LMM, MANOVA and NMDS were conducted in R version 3.2.1 (R Core Team 2014) using the functions lme() in the package “nlme”, manova() in the package “stats” and metaMDS() in the package “vegan”, respectively.
Results
Litter decomposition
On average, 70% of the initial dry mass of the leaf litter placed in the upper part of the cores remained at the end of the experiment. Rotation and defaunation did not significantly affect litter decomposition, however, the leaves decomposed faster in the cores of the + N than in those of the Ctr treatment (31.8% vs. 26.9%, F1,57 = 6.69, P = 0.01).
In general, the C/N ratio was higher in the cores of the Ctr than in those of the + N treatment with averages of 38.33 ± 0.22 vs. 33.74 ± 0.23 (F1,57 = 8.22, P = 0.005) for the L layer and 27.67 ± 0.26 vs. 25.39 ± 0.25 (F1,57 = 4.16, P = 0.04) for the F/H layer. Further, the C/N ratio of the L layer in defaunated cores exceeded that of the F/H layer with an average of 38.38 ± 0.29 vs. 33.69 ± 0.18 (F1,57 = 7.22, P = 0.009). Rotation of the cores did not significantly affect the C/N ratio of the L and F/H layer.
Concentrations of PO43− and available P (only measured in the F/H layer) did not differ significantly between treatments with averages of 0.21 ± 0.09 and 0.06 ± 0.02 mg g−1, respectively. However, the pH (only measured in F/H layer) in the + N treatment exceeded that in the Ctr treatment (3.94 vs. 3.79; F1,57 = 7.62, P = 0.007).
Arbuscular mycorrhizal fungi
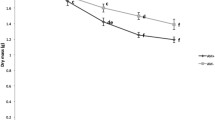
Generally, the concentration of NLFA 16:1ω5 in the F/H layer declined during the experiment from an overall mean of 33.38 ± 10.83 to 21.83 ± 5.11 nmol g−1 by the end of the experiment (F1,22 = 12.39, P = 0.001; Fig. 2a). By contrast, it stayed at the same level in the L layer (overall mean 16.08 ± 6.10 nmol g−1). Defaunation significantly reduced the concentration of NLFA 16:1ω5 in the F/H layer (F1,56 = 10.44, P = 0.002; Fig. 2b) but not in the L layer. Neither rotation nor N addition affected the concentrations of NLFA 16:1ω5 in the L and F/H layer.
Concentration of the AMF marker fatty acid in the L and F/H layers. Variations in the concentration of the AMF marker fatty acid 16:1ω5 in the L and F/H layers. a During exposure for 5 months in the field (initial = before exposure, final = at the end of experiment). b In defaunated (− Fauna) and non-defaunated cores (+ Fauna). Values are mean ± SE. **P < 0.01
Microbial biomass and respiration
In general, Cmic and BR in the L layer (overall means of 9308 ± 4031 µg Cmic g−1 dry weight (dw) and 47.63 ± 20.62 μl O2 g−1 dw h−1, respectively) exceeded that in the F/H layer (respective values of 44,394 ± 1685 µg Cmic g−1 dw and 43.42 ± 17.80 μl O2 g−1 dw h−1). In defaunated cores, Cmic in both the L and F/H layer was significantly reduced as compared to non-defaunated cores (F1,52 = 10.05, P = 0.002 and F1,52 = 20.81, P < 0.0001, respectively; Fig. 3a). BR also was significantly reduced in defaunated cores in the L layer (F1,52 = 7.61, P = 0.008; Fig. 3b), while in the F/H layer it was not significantly affected (overall mean of 39.92 ± 19.05 μl O2 g−1 dw h−1). Further, BR in the L layer in the + N treatment significantly exceeded that in the Ctr treatment (F1,52 = 6.52, P = 0.01), but this was not the case in the F/H layer (overall mean of 44.11 ± 15.70 μl O2 g−1 dw h−1). Also, Cmic was not significantly affected by N addition, neither in the L layer nor in the F/H layer (overall means of 9937 ± 4578 and 4555 ± 1607 µg Cmic g−1 dw, respectively). Further, rotation neither significantly affected Cmic (overall means of 9096 ± 4035 and 4346 ± 1699 µg Cmic g−1 dw in the L and F/H layer, respectively) nor BR (overall means of 47.48 ± 21.12 and 43.52 ± 21.77 μl O2 g−1 dw h−1 in the L and F/H layer, respectively).
Microbial community structure
The concentration of Gram-positive bacteria and saprotrophic fungi significantly changed during the experiment; Gram-positive bacteria significantly increased in the L layer from an overall mean of 80.80 ± 36.14 to 111.01 ± 32.13 nmol g−1 by the end of the experiment (F1,26 = 8.07, P = 0.008) and saprotrophic fungi significantly decreased in the F/H layer from an overall mean of 61.33 ± 12.23 to 52.31 ± 7.05 nmol g−1 by the end of the experiment (F1,26 = 6.42, P = 0.01).
The concentration of Gram-positive, Gram-negative and saprotrophic fungi markers in the L layer (overall means of 111.01 ± 39.59, 158.47 ± 59.74 and 200.54 ± 77.79 nmol g−1, respectively) exceeded that in the F/H layer (respective values of 76.77 ± 14.36, 94.38 ± 22.24 and 52.31 ± 13.93 nmol g−1). Gram-positive, Gram-negative and total bacterial PLFA markers were not significantly affected by any of the treatments, neither in the L nor in the F/H layer (Table 1). By contrast, in the L layer the concentration of the saprotrophic fungal markers 18:2ω6,9 and 18:1ω9 in non-rotated cores exceeded those in rotated cores (averages of 219.61 ± 78.33 and 181.48 ± 66.94 nmol g−1, respectively) while in the F/H layer the concentration did not differ significantly between rotated and non-rotated cores (overall mean of 54.21 ± 15.49 nmol g−1). Neither defaunation nor N addition affected the concentrations of the fungal markers in the L and F/H layer.
Microarthropods
Generally, microarthropods were more abundant in the F/H than in the L layer (Oribatida 17.85 ± 25.84 vs. 12.98 ± 12.62, Gamasina 3.32 ± 5.28 vs. 2.85 ± 3.21, Uropodina 4.07 ± 11.65 vs. 1.60 ± 3.01, Prostigmata 2.17 ± 2.91 vs. 1.28 ± 1.82, Astigmata 3.90 ± 12.66 vs. 0.98 ± 2.45, Collembola 6.70 ± 9.24 vs. 5.92 ± 7.01 ind. core−1). Overall, microarthropod numbers were lower in defaunated than in non-defaunated cores (Oribatida 3.82 ± 3.49 vs. 27.01 ± 23.53, Gamasina 2.81 ± 4.58 vs. 3.38 ± 4.16, Uropodina 0.56 ± 1.35 vs. 5.17 ± 11.72, Prostigmata 1.17 ± 1.86 vs. 2.30 ± 2.86, Astigmata 1.40 ± 3.17 vs. 3.52 ± 12.68, Collembola 4.48 ± 6.45 vs. 8.17 ± 9.32 ind. core−1). The number of Uropodina and Prostigmata was lower in both the L (averages of 0.65 ± 1.73 and 1.01 ± 1.79 ind. core−1, respectively) and the F/H layer of defaunated cores (averages of 0.46 ± 0.84 and 1.34 ± 1.94 ind. core−1, respectively). Further, in the L layer of non-defaunated cores the number of Astigmata exceeded that in defaunated cores with 1.67 ± 3.28 and 0.31 ± 0.82 ind. core−1, respectively. Also, in the F/H layer the number of Collembola in non-defaunated cores exceeded that in defaunated cores with 9.90 ± 11.17 and 3.50 ± 5.23 ind. core−1, respectively. Rotation generally slightly affected the number of microarthropods with the exception of Gamasina in the F/H layer of rotated cores which exceeded that in non-rotated cores with averages of 4.81 ± 6.86 and 1.84 ± 2.25 ind. core−1, respectively (Table 2).
In total, 60 species of Oribatida were identified (see S1 Table for full list of species). The three most common species of Oribatida associated with the L layer were Neoamerioppia rotunda, Cultroribula zicsii and Epieremulus granulatus, whereas in the F/H layer the three most common associated species were Rostrozetes foveolatus, Nanhermannia elegantissima and Scheloribates elegans. No Oribatida species exclusively occurred in any of the treatments. The PCA defined two main gradients of variation of the Oribatida communities, separating defaunated and non-defaunated cores and differentiating litter and soil layer, which together accounted for 57.8% of the total variation (Fig. 4). The first principal component axis (PC1) explained the majority of variability (48.7%) and was positively associated with non-defaunated soil cores, while the second principal component axis (PC2) explained only 9.1% and was associated with the soil layer. MANOVA performed with NMDS axes scores confirmed that defaunation strongly affected the Oribatida community in both the L and F/H layer (F6,49 = 6,61, P = 0.02; F6,47 = 6.52, P < 0.0001, respectively).
Principal components analysis of oribatid mite species. Principal components analysis (PCA) of oribatid mite species in control (Ctr), nitrogen addition (N), rotated (R), non-rotated (NR) defaunated (− F) and non-defaunated cores (+ F) in the L and F/H layer after 5 months of exposure in the field. The three most abundant species in the L layer are underlined and given in blue and the three most abundant species in the F/H layer are underlined and given in purple. Full species names are given in S1 Table
In general, both the richness and abundance of Oribatida were strongly affected by defaunation (Table 3), being lower in defaunated cores (2.90 ± 2.27 species core−1 and 4.50 ± 4.10 ind. core−1 in the L layer, and 2.21 ± 1.71 species core−1 and 3.15 ± 2.66 ind. core−1 in the F/H layer) than in non-defaunated cores (7.765 ± 3.01 species core−1 and 21.46 ± 12.58 ind. core−1 in the L layer, and 7.56 ± 4.03 species core−1 and 32.56 ± 30.06 ind. core−1 in the F/H layer). Neither rotation nor N addition affected the abundance or richness of microarthropods.
Discussion
Rotation of cores
Our findings do not provide evidence that AMF mycelia were reduced by regular rotation of the ingrowth cores. However, the AMF fatty acid marker inside the cores decreased during the experiment in the F/H layer. Kottke et al. (2004) observed extensive root development in the organic layer of the study sites and this likely favors the exploitation of nutrients by root-associated AMF. The soil inside ingrowth cores was separated from roots and the reduction in AMF marker concentration in the F/H layer material likely reflects the cutoff from the carbon supply from the plant to the fungal mycelium (Rillig 2004). The fact that the AMF marker fatty acid was not reduced by rotation of the ingrowth cores indicates that the AMF hyphae are only functioning in close association with roots without forming extensive extraradical mycelium. Further studies at the study site based on both fatty acids and microscopic inspection of AMF mycelia support this conclusion (Camenzind and Rillig 2013; Camenzind et al. 2014). Still, AMF may have affected the periphery of the non-rotated cores which are closer to living roots, with subtle effects to be detected when analyzing mixed samples from the complete core.
The reduced concentration of the AMF marker in the F/H layer neither was associated with a decline in Cmic nor in BR, indicating that microorganisms and microbial activity inside the cores were largely independent of AMF hyphae and their exudates based on plant carbon. Obviously, microorganisms in the F/H layer almost exclusively exploited dead organic matter resources comprising leaf litter and dead roots at later stages of decay. Notably, the reduction of AMF concentration also did not affect the abundance of soil microarthropods with the exception of Gamasina which significantly increased in the F/H layer of rotated cores. Gamasina typically live as predators hunting for other microarthropods, predominantly Collembola, as well as Nematoda (Koehler 1997; Dhooria 2016). Since Collembola were not affected by rotation of the cores, the increase in Gamasina due to rotation might have been due to increased nematode density in rotated cores. Interactions between nematodes and mycorrhiza have been assumed to be mutually inhibitory due to competition for space and food sources (Francl 1993; Pinochet et al. 1996; Borowicz 2001; Schouteden et al. 2015). However, the interactions are complex and as yet little understood in particular in tropical ecosystems (Hol and Cook 2005).
Oribatida richness, abundance and community composition were not significantly affected by rotation of the ingrowth cores. This indicates that they exclusively exploited resources inside the cores and this was independent of interruptions of fungal hyphae colonizing the cores. By contrast, Oribatida richness and abundance varied between soil horizons with both being considerably higher in the F/H than the L layer. The L layer was colonized by species typically occurring in the litter layer of the study site such as Cultroribula zicsii (Illig et al. 2005), whereas the F/H layer was colonized mostly by individuals of the genera Nanhermannia, Rostrozetes and Scheloribates typical inhabitants of F and H layers (Mitchell and Parkinson 1976; Illig et al. 2005). Unfortunately, little is known about the food sources of tropical Oribatida species, but presumably they comprise predominantly secondary decomposers feeding on microorganisms and microbial residues, with only few primary decomposers feeding on litter (Illig et al. 2005); this may explain the dominance of Oribatida in F/H material in the present study.
In contrast to the F/H layer, the concentration of AMF markers in the L layer did not significantly change during the experiment. In litter of an intermediate stage of decomposition, AMF are likely to compete for resources with saprotrophic fungi. Although the enzymatic capability of AMF typically is inferior to that of saprotrophic fungi, they effectively capture nutrients from decomposing litter material (Hodge et al. 2001; Camenzind and Rillig 2013), and this is most effective after saprotrophic microorganisms have started to decompose the litter (Posada et al. 2012). Previous work at our study site (Marian et al. 2019) also provided evidence that the presence of mycorrhiza suppresses the activity of saprotrophic microorganisms, thereby affecting the density of microarthropods such as Collembola. In our study, however, the abundance of microarthropods did not decline with the reduction of the AMF marker. Nevertheless, we assume that antagonistic interactions between AMF and saprotrophic fungi in the L layer persisted as colonization of litter by AMF did not decline during the experiment and this may have impacted other soil microorganisms (Krashevska et al. 2010). This conclusion is supported by the fact that saprotrophic fungi in the L layer in the non-rotated cores exceeded those in rotated cores pointing to stronger competition between saprotrophic fungi and AMF in non-rotated cores. However, part of the AMF marker might have originated from spores of AMF containing high amounts of AMF marker fatty acids (Olsson 1999), but this does not explain why AMF marker fatty acids declined in the F/H but not in the L layer during the experiment.
Defaunation
Arbuscular mycorrhizal fungi did not recover from defaunation in the F/H layer and this likely is related to excluding colonization of the cores by roots (see above). Similarly, Cmic did not recover from defaunation in the L and F/H layer during the experiment. Potentially, grazing by microarthropods contributed to the slow recovery. However, the abundances of all soil faunal groups investigated were strongly reduced in both the L and F/H layer of defaunated cores, pointing to restricted colonization of microarthropods from outside the ingrowth cores. This also indicates that the role of microarthropods in fragmenting organic material was reduced and thereby their contribution to the formation of new surface area facilitating microbial colonization (Seastedt 1984; Moore et al. 1988; Lussenhop 1992; Ruess and Lussenhop 2005). The restricted colonization by microarthropods together with the reduced Cmic in defaunated cores also might have been related to a decline in food availability due to a reduced input of root-derived resources in the cores. At our study sites, roots are concentrated in organic layers (Wilcke et al. 2002) and root-derived resources are increasingly recognized as being of fundamental importance in fueling soil food webs (Pollierer et al. 2007, 2012; Scheunemann et al. 2016; Zieger et al. 2017; Marian et al. 2019).
The slow recovery of Cmic and BR in both the L and F/H layer might have been due to low quality of litter. Nitrogen concentration in litter and litter decomposition is very low at our study site and this likely is responsible for the pronounced accumulation of organic matter in organic layers (Butenschoen et al. 2014; Marian et al. 2017). Low litter quality is associated with low nutrient mobilization during decomposition and therefore, to low supply of nutrients from the L to the F/H layer, and this is reflected by the decline in Cmic from the L layer to the F/H layer. Microbial biomass typically follows the stratified distribution of organic matter in the soil profile of forest ecosystems (Yang and Insam 1991; Wardle 1993). Presumably, both poor mineralization of nutrients from decomposing litter, and exclusion of roots and root-derived resources contributed to the restricted recovery of Cmic in defaunated soil cores. This is supported by the fact that BR stayed constant in the L and F/H layer in defaunated cores suggesting that microorganisms did not recover from the disturbance caused by defaunation.
Defaunation also significantly affected the community composition of Oribatida in both the L and F/H layers. Domes et al. (2007) showed that the eggs present in soil are sufficient to ensure establishment of a diverse community of Oribatida in organic layers, although Oribatida species differ in the speed they recover from disturbance. Therefore, the reduced diversity and abundance of Oribatida in defaunated cores might have been due to both reduced availability of resources as well as limited recovery and colonization of the cores by Oribatida from the surrounding soil, suggesting that colonization by Oribatida did not reach equilibrium during the 5 months of the experiment.
Nitrogen addition
The concentration of the AMF marker fatty acid was not significantly affected by N addition, neither in the L nor in the F/H layer. This contrasts results of previous studies (Camenzind et al. 2014) that AMF root colonization decreased due to N fertilization. The different findings are difficult to explain, but varying effects of N fertilization on AMF have been reported previously (reviewed in Treseder and Allen 2000; Rillig et al. 2003; Treseder 2004).
Earlier studies at our study site reported evidence that microorganisms benefited from N fertilization (Krashevska et al. 2010). Results of the present study support these findings, although the addition of N did not alter microbial biomass. However, the addition of N increased leaf litter decomposition rates and BR in the L layer suggesting that the availability of N limited microbial activity. This is supported by the lower C/N ratio in the L layer in the N addition treatment, reflecting that the addition of N improved litter quality for decomposer organisms. Overall, however, this suggests that nutrient dynamics inside the cores were largely independent of AMF hyphae and exudates.
The addition of N increased the pH in the F/H layer. Increase in pH may stimulate nutrient mobilization and this may result in increased microbial activity and microbial biomass (Thirukkumaran and Parkinson 2000; Vance and Chapin 2001). However, in our study N fertilization did not affect the microbial biomass levels and only little affected microbial activity in the F/H layer. Presumably, microorganisms in the F/H layer are not only limited by N but also by other nutrients. In fact, although increased pH may stimulate the mobilization of N, it may aggravate the limitation of P (Gallardo and Schlesinger 1994), and P supply is very low in the studied tropical montane rainforest (Krashevska et al. 2010; Homeier et al. 2012).
Conclusions
Results of this study suggest that in nutrient limited tropical montane rainforests AMF hyphae are in close association with roots and do not form extensive extraradical mycelia, indicating that interactions of AMF with other soil biota are restricted to the close vicinity of roots. In contrast to the F/H layer, AMF did not decline in the L layer during the experiment suggesting that interactions with other soil biota are concentrated in the L layer where AMF likely compete with saprotrophic microorganisms for litter-derived resources. The restricted recovery of microorganisms and microarthropods after defaunation inside the cores points to the importance of root-derived resources for fueling soil food webs. Unexpectedly, N addition did not affect AMF suggesting that N dynamics inside the cores were independent of mycorrhiza, despite litter N concentrations being increased and this likely was responsible for the increase in microbial respiration and decomposition due to N addition. Further research on interactions between AMF and other soil biota under field conditions is needed to improve our understanding of their role in structuring microbial and animal communities as well as their importance for decomposition processes in tropical forest ecosystems. The concentration of these interactions in the vicinity of roots and the litter layer, as suggested by results of the present study, pose particular challenges.
References
A’Bear AD, Jones TH, Boddy L (2014) Size matters: what have we learnt from microcosm studies of decomposer fungus-invertebrate interactions? Soil Biol Biochem 78:274–283
Anderson JPE, Domsch KH (1978) A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol Biochem 10:215–221
Bagyaraj D (2014) Ecology of arbuscular mycorrhizal fungi. In: Kharwar R, Upadhyay R, Dubey N, Raghuwanshi R (eds) Microbial diversity and biotechnology in food security. Springer, New Delhi, pp 133–146
Balogh J, Balogh P (1990) Oribatid mites of the Neotropical region II. Elsevier Science Publishers, Budapest
Balogh J, Balogh P (2002) Identification keys to the oribatid mites of the extra-holarctic regions I. Well-Press Publishing Limited, Hungary
Beck E, Ritcher M (2008) Ecological aspects of a biodiversity hotspot in the Andes of southern Ecuador. Biodivers Ecol Ser 2:195–217
Beck T, Joergensen RG, Kandeler E, Makeshin E, Nuss E, Oberholzer HR, Scheu S (1997) An inter-laboratory comparison of ten different ways of measuring soil microbial biomass C. Soil Biol Biochem 29:1023–1032
Bendix J, Homeier J, Cueva Ortiz E, Emck P, Breckle SW, Richter M, Beck E (2006) Seasonality of weather and tree phenology in a tropical evergreen mountain rain forest. Int J Biometeorol 50:370–384
Bödeker ITM, Lindahl BD, Olson A, Clemmensen KE (2016) Mycorrhizal and saprotrophic fungal guilds compete for the same organic substrates but affect decomposition differently. Funct Ecol 30:1967–1978
Borowicz VA (2001) Do arbuscular mycorrhizal fungi alter plant-pathogen relations? Ecology 82:3057–3068
Bray RH, Kurtz LT (1945) Determination of total, organic, and available forms of phosphorus in soils. Soil Sci 59:39–46
Brundrett MC, Tedersoo L (2018) Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol 220:1108–1115
Butenschoen O, Krashevska V, Maraun M, Marian F, Sandmann D, Scheu S (2014) Litter mixture effects on decomposition in tropical montane rainforests vary strongly with time and turn negative at later stages of decay. Soil Biol Biochem 77:121–128
Camenzind T, Rillig MC (2013) Extraradical arbuscular mycorrhizal fungal hyphae in an organic tropical montane forest soil. Soil Biol Biochem 64:96–102
Camenzind T, Hempel S, Homeier J, Horn S, Velescu A, Wilcke W, Rillig MC (2014) Nitrogen and phosphorus additions impact arbuscular mycorrhizal abundance and molecular diversity in a tropical montane forest. Glob Chang Biol 20:3646–3659
Cardoso IM, Kuyper TW (2006) Mycorrhizas and tropical soil fertility. Agric Ecosyst Environ 116:72–84
Dalling JW, Heineman K, Lopez OR, Wright SJ, Turner BL (2016) Nutrient availability in tropical rain forests: the paradigm of phosphorus limitation. In: Goldstein G, Editors LSS (eds) Tropical tree physiology. Springer, New York, pp 261–273
Dhooria MS (2016) Fundamentals of applied acarology. Fundamentals of applied acarology. Springer, Singapore, pp 1–470
Domes K, Scheu S, Maraun M (2007) Resources and sex: soil re-colonization by sexual and parthenogenetic oribatid mites. Pedobiologia (Jena) 51:1–11
Finlay RD (2004) Mycorrhizal fungi and their multifunctional roles. Mycologist 18:91–96
Francl LJ (1993) Interactions of nematodes with mycorrhizae and mycorrhizal fungi. In: Khan MW (ed) Nematode Interactions. Springer, Dordrecht, pp 203–216
Franklin E, Hayek T, Fagundes EP, Silva LL (2004) Oribatid mite (Acari: Oribatida) contribution to decomposition dynamic of leaf litter in primary forest, second growth, and polyculture in the Central Amazon. Braz J Biol 64:59–72
Frostegård Å, Bååth E (1996) The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol Fertil Soils 22:59–65
Frostegård A, Tunlid A, Bååth E (1993) Phospholipid Fatty Acid composition, biomass, and activity of microbial communities from two soil types experimentally exposed to different heavy metals. Appl Environ Microbiol 59:3605–3617
Gallardo A, Schlesinger WH (1994) Factors limiting microbial biomass in the mineral soil and forest floor of a warm-temperate forest. Soil Biol Biochem 26:1409–1415
Gange AC, Brown VK (2002) Actions and interactions of soil invertebrates and arbuscular mycorrhizal fungi in affecting the structure of plant communities. In: van der Heijden MGA, Sanders IR (eds) Mycorrhizal ecology. Ecological studies (analysis and synthesis). Springer, Berlin, pp 321–344
Gryndler M (2000) Interactions of arbuscular mycorrhizal fungi with other soil organisms. In: Kapulnik Y, Douds DD (eds) Arbuscular mycorrhizas: physiology and function. Springer, Dordrecht, pp 239–262
Hammer M (1958) Investigations on the Oribatid fauna of the Andes Mountains I. The Argentine and Bolivia. Biol Skr Dan Vid Selsk 10(1):1–129
Hammer M (1961) Investigations on the Oribatid fauna of the Andes Mountains II. The Argentine and Bolivia. Biol Skr Dan Vid Selsk 13(1):1–157
Hodge A, Campbell CD, Fitter AH (2001) An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 413:297–299
Hol WHG, Cook R (2005) An overview of arbuscular mycorrhizal fungi-nematode interactions. Basic Appl Ecol 6:489–503
Homeier J, Breckle S, Günter S, Rollenbeck RT, Homeier J, Breckle S, Günter S, Rollenbeck R, Leuschner C (2010) Tree diversity, forest structure and productivity along altitudinal and topographical gradients in a species-rich Ecuadorian montane rain forest. Biotropica 42:140–148
Homeier J, Hertel D, Camenzind T, Cumbicus NL, Maraun M, Martinson GO, Poma LN, Rillig MC, Sandmann D, Scheu S, Veldkamp E, Wilcke W, Wullaert H, Leuschner C (2012) Tropical Andean forests are highly susceptible to nutrient inputs-rapid effects of experimental N and P addition to an Ecuadorian montane forest. PLoS One 7:e47128
Illig J, Langel R, Norton RA, Scheu S, Maraun M (2005) Where are the decomposers? Uncovering the soil food web of a tropical montane rain forest in southern Ecuador using stable isotopes (15 N). J Trop Ecol 21:589–593
Johansson JF, Paul LR, Finlay RD (2004) Microbial interactions in the mycorrhizosphere and their significance for sustainable agriculture. FEMS Microbiol Ecol 48:1–13
Johnson NC (2010) Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol 185:631–647
Johnson D, Leake JR, Read DJ (2001) Novel in-growth core system enables functional studies of grassland mycorrhizal mycelial networks. New Phytol 152:555–562
Kempson D, Lloyd M, Ghelardi R (1963) A new extractor for woodland litter. Pedobiologia (Jena) 3:1–21
Koehler HH (1997) Mesostigmata (Gamasina, Uropodina), efficient predators in agroecosystems. Agric Ecosyst Environ 62:105–117
Kottke I, Beck A, Oberwinkler F, Homeier J, Neill D (2004) Arbuscular endomycorrhizas are dominant in the organic soil of a neotropical montane cloud forest. J Trop Ecol 20:125–129
Krashevska V, Maraun M, Ruess L, Scheu S (2010) Carbon and nutrient limitation of soil microorganisms and microbial grazers in a tropical montane rain forest. Oikos 119:1020–1028
Lehmann A, Leifheit EF, Rillig MC (2017) Mycorrhizas and soil aggregation. In: Collins Johnson N, Gehring C, Jansa J (eds) Mycorrhizal mediation of soil: fertility, structure, and carbon storage. Elsevier, Amsterdam, pp 241–262
Leifheit EF, Verbruggen E, Rillig MC (2014) Rotation of hyphal in-growth cores has no confounding effects on soil abiotic properties. Soil Biol Biochem 79:78–80
Lussenhop J (1992) Mechanisms of microarthropod–microbial interactions in soil. Adv Ecol Res 23:1–33
Macfadyen A (1961) Improved funnel-type extractors for soil arthropods. J Anim Ecol 30:171–184
Maraun M, Martens H, Migge S, Theenhaus A, Scheu S (2003) Adding to “the enigma of soil animal diversity”: fungal feeders and saprophagous soil invertebrates prefer similar food substrates. Eur J Soil Biol 39:85–95
Marian F, Sandmann D, Krashevska V, Maraun M, Scheu S (2017) Leaf and root litter decomposition is discontinued at high altitude tropical montane rainforests contributing to carbon sequestration. Ecol Evol 7:6432–6443
Marian F, Brown L, Sandmann D, Maraun M, Scheu S (2019) Roots, mycorrhizal fungi and altitude as determinants of litter decomposition and soil animal communities in tropical montane rainforests. Plant Soil 438:1–8
Miransari M (2011) Interactions between arbuscular mycorrhizal fungi and soil bacteria. Appl Microbiol Biotechnol 89:917–930
Mitchell MJ, Parkinson D (1976) Ecological Society of America Fungal Feeding or Oribatid Mites (Acari: Cryptostigmata) in an Aspen Woodland Soil. Ecology 57:302–312
Moore JC, Walter DE, Hunt HW (1988) Arthropod regulation of micro and mesobiota in belowground detrital food webs. Annu Rev Entomol 33:419–435
Ngosong C, Gabriel E, Ruess L (2014) Collembola grazing on arbuscular mycorrhiza fungi modulates nutrient allocation in plants. Pedobiologia (Jena) 57:171–179
Nottingham AT, Turner BL, Winter K, Chamberlain PM, Stott A, Tanner EVJ (2013) Root and arbuscular mycorrhizal mycelial interactions with soil microorganisms in lowland tropical forest. FEMS Microbiol Ecol 85:37–50
Nuccio EE, Hodge A, Pett-ridge J, Herman DJ, Weber PK, Firestone MK (2013) An arbuscular mycorrhizal fungus significantly modifies the soil bacterial community and nitrogen cycling during litter decomposition. Environ Microbiol 15:1870–1881
Olsson PA (1999) Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil. FEMS Microbiol Ecol 29:303–310
Olsson PA, Bååth E, Jakobsen I, Söderström B (1995) The use of phospholipid and neutral lipid fatty acids to estimate biomass of arbuscular mycorrhizal fungi in soil. Mycol Res 99:623–629
Öpik M, Moora M, Liira J, Zobel M (2006) Composition of root-colonizing arbuscular mycorrhizal fungal communities in different ecosystems around the globe. J Ecol 94:778–790
Pinochet J, Calvet C, Camprubí A, Fernández C (1996) Interactions between migratory endoparasitic nematodes and arbuscular mycorrhizal fungi in perennial crops: a review. Plant Soil 185:183–190
Pollierer MM, Langel R, Körner C, Maraun M, Scheu S (2007) The underestimated importance of belowground carbon input for forest soil animal food webs. Ecol Lett 10:729–736
Pollierer MM, Dyckmans J, Scheu S, Haubert D (2012) Carbon flux through fungi and bacteria into the forest soil animal food web as indicated by compound-specific 13C fatty acid analysis. Funct Ecol 26:978–990
Posada RH, Madriñan S, Rivera EL (2012) Relationships between the litter colonization by saprotrophic and arbuscular mycorrhizal fungi with depth in a tropical forest. Fungal Biol 116:747–755
R Core Team (2014) R: A language and environment for statistical computing. http://www.r-project.org/
Read DJ, Perez-Moreno J (2003) Mycorrhizas and nutrient cycling in ecosystems—a journey towards relevance? New Phytol 157:475–492
Renker C, Otto P, Schneider K, Zimdars B, Maraun M, Buscot F (2005) Oribatid mites as potential vectors for soil microfungi: study of mite-associated fungal species. Microb Ecol 50:518–528
Richter M, Diertl KH, Emck P, Peters T, Beck E (2009) Reasons for an outstanding plant diversity in the tropical Andes of Southern Ecuador. Landsc Online 12:1–35
Rillig MC (2004) Arbuscular mycorrhizae and terrestrial ecosystem processes. Ecol Lett 7:740–754
Rillig MC, Treseder KK, Allen MF (2003) Global change and mycorrhizal fungi. In: van der Heijden MGA, Sanders IR (eds) Mycorrhizal ecology. Springer, Berlin, pp 135–160
Ruess L, Chamberlain PM (2010) The fat that matters: soil food web analysis using fatty acids and their carbon stable isotope signature. Soil Biol Biochem 42:1898–1910
Ruess L, Lussenhop J (2005) Trophic interactions of fungi and animals. In: Dighton J, Oudemans P, White J (eds) The fungal community: its organization and role in the ecosystem. CRC, Boca Raton, pp 581–598
Scheu S (1992) Automated measurement of the respiratory response of soil microcompartments: active microbial biomass in earthworm faeces. Soil Biol Biochem 24:1113–1118
Scheunemann N, Pausch J, Digel C, Kramer S, Scharroba A, Kuzyakov Y, Kandeler E, Ruess L, Butenschoen O, Scheu S (2016) Incorporation of root C and fertilizer N into the food web of an arable field: variations with functional group and energy channel. Food Webs 9:39–45
Schneider K, Renker C, Maraun M (2005) Oribatid mite (Acari, Oribatida) feeding on ectomycorrhizal fungi. Mycorrhiza 16:67–72
Schouteden N, De Waele D, Panis B, Vos CM (2015) Arbuscular mycorrhizal fungi for the biocontrol of plant-parasitic nematodes: a review of the mechanisms involved. Front Microbiol 6:1–12
Seastedt TR (1984) The role of microarthropods in decomposition and mineralization processes. Annu Rev Entomol 29:25–46
Sheldrake M, Rosenstock NP, Mangan S, Revillini D, Sayer EJ, Olsson PA, Verbruggen E, Tanner EVJ, Turner BL, Wright SJ (2018) Responses of arbuscular mycorrhizal fungi to long-term inorganic and organic nutrient addition in a lowland tropical forest. ISME J 12:2433–2445
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academy Press, London
Subías LS (2018) Listado sistemático, sinonímico y biogeográfico de los ácaros oribátidos (Acariformes: Oribatida) del mundo (Excepto fósiles). p 605. http://www.ucm.es/info/zoo/Artropodos/Catalogo.pdf. Accessed Feb 2018
Svenningsen NB, Watts-Williams SJ, Joner EJ, Battini F, Efthymiou A, Cruz-Paredes C, Nybroe O, Jakobsen I (2018) Suppression of the activity of arbuscular mycorrhizal fungi by the soil microbiota. ISME J 12:1296–1307
Ter Braak C, Smilauer P (2012) Canoco reference manual and user’s guide: Software for ordination (version 5.0)
Thirukkumaran CM, Parkinson D (2000) Microbial respiration, biomass, metabolic quotient and litter decompostion. Soil Biol Biochem 32:59–66
Toljander JF, Artursson V, Paul LR, Jansson JK, Finlay RD (2007) Attachment of different soil bacteria to arbuscular mycorrhizal fungal extraradical hyphae is determined by hyphal vitality and fungal species. FEMS Microbiol Lett 254:34–40
Treseder K (2004) A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol 164:347–355
Treseder KK, Allen MF (2000) Mycorrhizal fungi have a potential role in soil carbon storage under elevated CO and nitrogen deposition. New Phytol 147:189–200
Treseder KK, Allen MF (2002) Direct nitrogen and phosphorus limitation of arbuscular mycorrhizal fungi: a model and field test. New Phytol 155:507–515
Vance ED, Chapin FS (2001) Substrate limitations to microbial activity in taiga forest floors. Soil Biol Biochem 33:173–188
Vašutová M, Mleczko P, López-García A, Maček I, Boros G, Ševčík J, Fujii S, Hackenberger D, Tuf IH, Hornung E, Páll-Gergely B, Kjøller R (2019) Taxi drivers: the role of animals in transporting mycorrhizal fungi. Mycorrhiza. https://doi.org/10.1007/s00572-019-00906-1
Wallander H, Ekblad A, Godbold DL, Johnson D, Bahr A, Baldrian P, Björk RG, Kieliszewska-Rokicka B, Kjøller R, Kraigher H, Plassard C, Rudawska M (2013) Evaluation of methods to estimate production, biomass and turnover of ectomycorrhizal mycelium in forests soils—a review. Soil Biol Biochem 57:1034–1047
Wardle DA (1993) Changes in the microbial biomass and metabolic quotient during leaf litter succession in some New Zealand forest and scrubland ecosystems. Funct Ecol 7:346
Wilcke W, Yasin S, Abramowski U, Valarezo C, Zech W (2002) Nutrient storage and turnover in organic layers under tropical montane rain forest in Ecuador. Eur J Soil Sci 53:15–27
Wullaert H, Homeier J, Valarezo C, Wilcke W (2010) Response of the N and P cycles of an old-growth montane forest in Ecuador to experimental low-level N and P amendments. For Ecol Manage 260:1434–1445
Yang JC, Insam H (1991) Microbial biomass and relative contributions of bacteria and fungi in soil beneath tropical rain forest, Hainan Island, China. J Trop Ecol 7:385–393
Zelles L (1997) Phospholipid fatty acid profiles in selected members of soil microbial communities. Chemosphere 35:275–294
Zelles L (1999) Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: a review. Biol Fertil Soils 29:111–129
Zieger SL, Ammerschubert S, Polle A, Scheu S (2017) Root-derived carbon and nitrogen from beech and ash trees differentially fuel soil animal food webs of deciduous forests. PLoS One 12:1–14
Acknowledgements
We would like to thank the Deutsche Forschungsgemeinschaft (DFG FOR816) for financial support. Further, we thank the Ministerio de Ambiente del Ecuador and the Universidad Técnico Particular de Loja (UTPL) for the research permits and the center Naturaleza y Cultura Internacional (NCI) to allow us to work in the San Francisco reserve. We thank Miguel Velez Espinoza for his collaboration during the field work and Odette Gonzalez Macé for helpful comments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sánchez-Galindo, L.M., Camenzind, T., Maraun, M. et al. Impacts of core rotation, defaunation and nitrogen addition on arbuscular mycorrhizal fungi, microorganisms and microarthropods in a tropical montane rainforest. Trop Ecol 60, 350–361 (2019). https://doi.org/10.1007/s42965-019-00038-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42965-019-00038-9