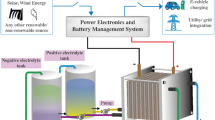
The vanadium redox flow battery (VRFB) has been widely used in large-scale energy storage areas due to the advantages of long lifespan and high safety. However, the high preparation cost of vanadium electrolyte limits the large-scale commercial application of VRFB. In this work, a new efficient cleaner short process for preparing V3.5+ vanadium electrolyte was proposed. With ammonium metavanadate (NH4VO3) and ammonia as raw materials, a mixture of V(IV) and V(III) vanadium oxides was produced by a roasting process directly, which could be further dissolved to obtain the V3.5+ vanadium electrolyte product. The reaction mechanism of ammonia reduction of NH4VO3 was studied. It was found that the valence of vanadium in the reduction product decreased with the increase of the reaction temperature. The influence of roasting and dissolving parameters was also investigated, and it was found that increasing the reaction temperature and the ammonia flow rate would promote the reduction of NH4VO3, and keeping the reaction temperature and ammonia flow rate constant, extending the reaction time would also increase the reduction degree of NH4VO3. And, the solubility of the reduction product showed a trend of increase first and then decrease with raising sulfuric acid concentration in the solvent. Under the reaction temperature of 480 °C, ammonia flow rate of 100 mL·min−1, reaction time of 50 min, and dissolved solution of 20% sulfuric acid, a V3.5+ electrolyte can be obtained.




Similar content being viewed by others
Data availability
The data generated during and/or analysed in this article are available from the corresponding author on reasonable request.
References
Qu D, Yang F, Fan L, Feng X, Ma J. Review of vanadium redox flow battery technology. J Jilin Univer (Engineering Edition). 2022;52(01):1. https://doi.org/10.13229/j.cnki.jdxbgxb20210047.
Fan Q, Tian Z, Gui Y, Wu Y, Li G. Research Progress on preparation methods and application properties of VRFB electrolyte. China Molybdenum Industr. 2022;46(01):44. https://doi.org/10.13384/j.cnki.cmi.1006-2602.2022.01.009.
Yi-Han Fu, Yuan-You Peng, Lei Zhao, Tian-Qi He, Mei-Mei Yuan, Hao Dang, Rui Liu, Fen Ran. Vanadium nitride quantum dots@carbon skeleton anode material synthesized via in situ oxidation initiation strategy. Tungsten. https://doi.org/10.1007/s42864-023-00246-w
Guo Y, Huang J, Feng J. Research progress in preparation of electrolyte for all-vanadium redox flow battery. J Ind Eng Chem. 2023;118:33. https://doi.org/10.1016/j.jiec.2022.11.037.
Alotto P, Guarnieri M, Moro F. Redox flow batteries for the storage of renewable energy: a review. Renew Sustain Energy Rev. 2014;29:325. https://doi.org/10.1016/j.rser.2013.08.001.
Sum E, Rychcik M, Skyllas-Kazacos M. Investigation of the V(V)/V(IV) system for use in the positive half-cell of a redox battery. J Power Sources. 1985;16(2):85. https://doi.org/10.1016/0378-7753(85)80082-3.
Yang Y, Zhang Y, Huang J, Liu T, Zheng Q. Selection and performance of reductant in electrolyte of vanadium battery prepared by chemical reduction method. Chem Indust Eng Prog. 2017;36(01):274. https://doi.org/10.16085/j.issn.1000-6613.2017.01.034.
Yang L. Preparation and mechanism of high purity vanadium oxysulfate for vanadium battery based on solvent extraction. Master Thesis, Guangzhou: Guangdong University of Technology, 2021.
Chu SB, Zhou LZ, Wang ZM. Study on thermal decomposition of ammonium metavanadate. Chemi Metallurgy. 1991;01:69.
Hryha E, Rutqvist E, Nyborg L. Stoichiometric vanadium oxides studied by XPS. Surf Interface Anal. 2012;44(8):1022. https://doi.org/10.1002/sia.3844.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, C., Li, LJ. & Du, H. Cleaner production of 3.5 valent vanadium electrolyte from ammonium metavanadate by ammonia reduction-sulfuric acid dissolution method. Tungsten 6, 555–560 (2024). https://doi.org/10.1007/s42864-023-00249-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42864-023-00249-7