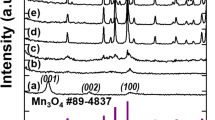
Abstract
Molybdenum trioxide (MoO3) is regarded as a promising electrode material for lithium (Li)-ion batteries because of its unique layered structure with a high theoretical capacity (~ 1117 mAh·g−1). Till now, numerous researches have focused on tuning MoO3 morphology to improve its electrochemical performance. However, the fabrication of MoO3 with a two-dimensional (2D) nanosheet clusters structure has yet been achieved. Here, we report a facile one-step solvothermal method to prepare MoO3 nanosheets, of which the morphology can be facilely tuned via the dose of hydrogen peroxide. Both the experimental results and theoretical calculation suggest that the resultant 2D nanosheets structure could reduce the diffusion paths, which is beneficial for the intercalation of Li-ion. As a result, the nanosheets assembled Li-ion battery has a reversible specific capacity of 756.1 mAh·g−1 at 0.5 A·g−1, and maintained at 214.7 mAh·g−1 after 600 cycles at a current density of 1 A·g−1 with the Coulombic efficiency as high as 97.17%.








Similar content being viewed by others
Change history
21 August 2021
A Correction to this paper has been published: https://doi.org/10.1007/s42864-021-00115-4
References
Zeng G, Zhao J, Feng C, Chen D, Meng Y, Boateng B, Lu N, He W. Flame-retardant bilayer separator with multifaceted van der Waals interaction for lithium-ion batteries. ACS Appl Mater Inter. 2019;11(29):26402.
Zhou H, Xia X, Lv P, Zhang J, Hou X, Zhao M, Ao K, Wang D, Lu K, Qiao H, Zimniewska M, Wei Q. C@TiO2 /MoO3 composite nanofibers with 1T phase MoS2 manograin dopant and stabilized interfaces as anodes for Li- and Na-ion batteries. Chemsuschem. 2018;11(23):4060.
Liu X, Liu Y, Yan X, Lan JL, Yu Y, Yang X. Ultrafine MoO3 nanoparticles embedded in porous carbon nanofibers as anodes for high-performance lithium-ion batteries. Mater Chem Front. 2019;3(1):120.
Tojo T, Tawa H, Oshida N, Inada R, Sakurai Y. Electrochemical characterization of a layered α-MoO3 as a new cathode material for calcium ion batteries. J Electroanal Chem. 2018;825:51.
Naresh N, Jena P, Satyanarayana N. Facile synthesis of MoO3/rGO nanocomposite as anode materials for high performance lithium-ion battery applications. J Alloy Compd. 2019;810: 151920.
Wu X, Zhao SX, Yu LQ, Li JW, Zhao EL, Nan CW. Lithium storage behavior of MoO3-P2O5 glass as cathode material for Li-ion batteries. Electrochim Acta. 2019;297:872.
Wang P, Chen Z, Ji Z, Feng Y, Wang J, Liu J, Hu M, Wang H, Gan W, Huang Y. A flexible aqueous Al ion rechargeable full battery. Chem Eng J. 2019;2019(373):580.
Oh SH, Park SM, Kang DW, Kang YC, Cho JS. Fibrous network of highly integrated carbon nanotubes/MoO3 composite bundles anchored with MoO3 nanoplates for superior lithium ion battery anodes. J Ind Eng Chem. 2020;83:438.
Cao L, He J, Li J, Yan J, Huang J, Qi Y, Feng L. Surface tiny grain-dependent enhanced rate performance of MoO3 nanobelts with pseudo capacitance contribution for lithium-ion battery anode. J Power Sour. 2018;392:87.
Sun H, Hanlon D, Dinh DA, Boland JB, Del Rio Castillo AE, Di Giovanni C, Ansaldo A, Pellegrini V, Coleman JN, Bonaccorso F. Carbon nanotubes-bridged molybdenum trioxide nanosheets as high performance anode for lithium ion batteries. 2D Mater. 2017;5(1): 015024.
Zheng C, Luo N, Huang S, Wu W, Huang H, Wei M. Nanocomposite of Mo2N quantum dots@MoO3@nitrogen-doped carbon as a high-performance anode for lithium-ion batteries. ACS Sustain Chem Eng. 2019;7(12):10198.
Cheng X, Li Y, Sang L, Ma J, Shi H, Liu X, Lu J, Zhang Y. Boosting the electrochemical performance of MoO3 anode for long-life lithium ion batteries: dominated by an ultrathin TiO2 passivation layer. Electrochim Acta. 2018;269:241.
Hu Z, Zhang X, Peng C, Lei G, Li Z. Pre-intercalation of potassium to improve the electrochemical performance of carbon-coated MoO3 cathode materials for lithium batteries. J Alloy Compd. 2020;826: 154055.
Wang S, Zhang H, Zhang D, Ma Y, Bi X, Yang S. Vertically oriented growth of MoO3 nanosheets on graphene for superior lithium storage. J Mater Chem A. 2018;6(2):672.
Yang C, Liu X, Yang Z, Gu L, Yu Y. Improvement of lithium storage performance of molybdenum trioxide by a synergistic effect of surface coating and oxygen vacancies. Adv Mater Interfaces. 2016;3(22):1600730.
Jiang Y, Sun M, Ni J, Li L. Ultrastable sodium storage in MoO3 nanotube arrays enabled by surface phosphorylation. ACS Appl Mater Inter. 2019;11(41):37761.
Sun S, Xia Q, Liu J, Xu J, Zan F, Yue J, Savilov SV, Lunin VV, Xia H. Self-standing oxygen-deficient α-MoO3−x nanoflake arrays as 3D cathode for advanced all-solid-state thin film lithium batteries. J Materiomics. 2019;5(2):229.
Fjellvåg ØS, Ruud A, Sønsteby HH, Nilsen O, Fjellvåg H. Crystallization, phase stability, and electrochemical performance of β-MoO3 thin films. Cryst Growth Des. 2020;20(6):3861.
Chen Z, Liu Y, Zhang H, Ding S, Wang T, Zhang M. In-situ phase transition to form porous h-MoO3@C nanofibers with high stability for Li+/Na+ storage. Sci China Mater. 2017;60(8):755.
Wu H, Zhou S, Tseng C, Qin M, Shiue A, Chu A, Cao Z, Jia B, Qu X. One-pot solution combustion synthesis of crystalline and amorphous molybdenum trioxide as anode for lithium-ion battery. J Am Ceram Soc. 2021;104(2):1102.
Wu C, Xie H, Li D, Liu D, Ding S, Tao S, Chen H, Liu Q, Chen S, Chu W, Zhang B, Song L. Atomically intercalating tin ions into the interlayer of molybdenum oxide nanobelt toward long-cycling lithium battery. J Phys Chem Lett. 2018;9(4):817.
Qu G, Wang J, Liu G, Tian B, Su C, Chen Z, Rueff JP, Wang Z. Vanadium doping enhanced electrochemical performance of molybdenum oxide in lithium-ion batteries. Adv Funct Mater. 2019;29(2):1805227.
Wang B, Ang EH, Yang Y, Zhang Y, Geng H, Ye M, Li CC. Interlayer engineering of molybdenum trioxide toward high-capacity and stable sodium ion half/full batteries. Adv Funct Mater. 2020;30(28):2001708.
Zhou J, Lin N, Wang L, Zhang K, Zhu Y, Qian Y. Synthesis of hexagonal MoO3 nanorods and a study of their electrochemical performance as anode materials for lithium-ion batteries. J Mater Chem A. 2015;3(14):7463.
Ahmed B, Shahid M, Nagaraju DH, Anjum DH, Hedhili MN, Alshareef HN. Surface passivation of MoO3 nanorods by atomic layer deposition toward high rate durable Li ion battery anodes. ACS Appl Mater Inter. 2015;7(24):13154.
Xie S, Cao D, She Y, Wang H, Shi JW, Leung MKH, Niu C. Atomic layer deposition of TiO2 shells on MoO3 nanobelts allowing enhanced lithium storage performance. Chem Commun. 2018;54(56):7782.
Huang C, Pi C, Zhang X, Ding K, Qin P, Fu J, Peng X, Gao B, Chu PK, Huo K. In situ synthesis of MoP nanoflakes intercalated N-doped graphene nanobelts from MoO3-amine hybrid for high-efficient hydrogen evolution reaction. Small. 2018;14(25):1800667.
Cao D, Dai Y, Xie S, Wang H, Niu C. Pyrolytic synthesis of MoO3 nanoplates within foam-like carbon nanoflakes for enhanced lithium ion storage. J Colloid Interf Sci. 2018;514:686.
He X, Zhang H, Zhao X, Zhang P, Chen M, Zheng Z, Han Z, Zhu T, Tong Y, Lu X. Stabilized molybdenum trioxide nanowires as novel ultrahigh-capacity cathode for rechargeable zinc ion battery. Adv Sci. 2019;6(14):1900151.
Zhao G, Zhang N, Sun K. Electrochemical preparation of porous MoO3 film with a high rate performance as anode for lithium ion batteries. J Mater Chem A. 2013;1(2):221.
Burton BP, Stokes HT, Van D. First principles phase diagram calculations for the octahedral-interstitial system ZrOX, 0 ≤ X ≤ 1/2. J Phys Soc Jpn. 2012;81(1): 014004.
Forrest K, Pham T, Nugent P, Burd SD, Space B. Examining the effects of different ring configurations and equatorial fluorine atom positions on CO2 sorption in [Cu(bpy)2SiF6]. Cryst Growth Des. 2013;13(10):4542.
Blochl PE. Projector augmented-wave method. Phys Rev B. 1994;50:17953.
Kresse G, Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys Rev B. 1999;59(3):1758.
Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys Rev Lett. 1996;77(18):3865.
Kresse G, Furthmüller J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp Mater Sci. 1996;6(1):5.
Rudy C, Willock DJ. The (010) surface of alpha-MoO3, a DFT + U study. Phys Chem Chem Phys. 2005;7(22):3819.
Wu K, Zhan J, Xu G, Zhang C, Pan D, Wu M. MoO3 nanosheet arrays as superior anode materials for Li- and Na-ion batteries. Nanoscale. 2018;10(34):16040.
Luo L, Qin X, Wu J, Liang G, Li Q, Liu M, Kang F, Chen G, Li B. An interwoven MoO3@CNT scaffold interlayer for high-performance lithium-sulfur batteries. J Mater Chem A. 2018;6(18):8612.
Yang W, Wei Y, Chen Q, Qin S, Zuo J, Tan S, Zhai P, Cui S, Wang H, Jin C, Xiao J, Liu W, Shang J, Gong Y. A MoO3/MoO2-CP self-supporting heterostructure for modification of lithium-sulfur batteries. J Mater Chem A. 2020;8(31):15816.
Hu C, Shu H, Shen Z, Zhao T, Liang P, Chen X. Hierarchical MoO3/SnS2 core-shell nanowires with enhanced electrochemical performance for lithium-ion batteries. Phys Chem Chem Phys. 2018;20(25):17171.
Chen X, Huang Y, Zhang K. α-MoO3 nanorods coated with SnS2 nano sheets core-shell composite as high-performance anode materials of lithium ion batteries. Electrochim Acta. 2016;222:956.
Qi D, Chu H, Wang K, Li X, Huang J. A cellulose derived nanotubular MoO3/SnO2 composite with superior lithium storage properties. Chem Sel. 2018;3(44):12469.
Qi D, Zhang Y, Jia D, Huang J. Self-assembly approach for synthesis of nanotubular molybdenum trioxide/titania composite anode for lithium-ion batteries. Energy Technol Ger. 2017;5(11):2015.
Cho JS. Large scale process for low crystalline MoO3-carbon composite microspheres prepared by one-step spray pyrolysis for anodes in lithium-ion batteries. Nanomater Basel. 2019;9(4):539.
Wu QL, Zhao SX, Yu L, Yu LQ, Zheng XX, Wei G. In situ synthesis and electrochemical performance of MoO3-x nanobelts as anode materials for lithium-ion batteries. Dalton T. 2019;48(34):12832.
Varghese AP, Gangaja B, Nair S, Santhanagopalan D. New Li-ion battery full-cells: MoO3 nanobelts as high energy density electrode. Mater Res Express. 2019;6(7): 075003.
Liu H, Chen X, Deng L, Ding M, Li J, He X. Perpendicular growth of few-layered MoS2 nanosheets on MoO3 nanowires fabricated by direct anion exchange reactions for high-performance lithium-ion batteries. J Mater Chem A. 2016;4(45):17764.
Ersan F, Ozaydin HD, Gökoğlu G, Aktürk E. Theoretical investigation of lithium adsorption, diffusion and coverage on MX2 (M=Mo, W; X=O, S, Se, Te) monolayers. Appl Surf Sci. 2017;425:301.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 51972200).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wei, H., Yang, HY., Zhang, XQ. et al. Hydrogen peroxide enabled two-dimensional molybdenum trioxide nanosheet clusters for enhanced surface Li-ion storage. Tungsten 3, 338–347 (2021). https://doi.org/10.1007/s42864-021-00093-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42864-021-00093-7