Abstract
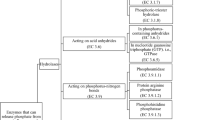
Enzymes adsorbed on clay minerals and soil colloids may exhibit lower activities compared to those of free enzymes. A particular toxic metal may affect the activity of the adsorbed enzyme less critically than that of the free form, however. This information is necessary for predicting catalytic performances of clay-immobilized enzymes in natural soils as well as in food, pharmaceutical, and chemical systems. The objective of the present study was to find out how adsorption on palygorskite and sepiolite minerals modifies the catalytic activity and the Michaelis–Menten kinetics of alkaline phosphatase (ALP). Inhibition kinetics of adsorbed ALP by Cd was also compared to that of the free enzyme. The results revealed that the affinity to the substrate and the maximum reaction velocity of ALP decreased upon adsorption on the fibrous clay minerals. The ALP adsorbed maintained a reasonably high activity recovery (AR) compared to the free enzyme. The AR of the adsorbed ALP ranged from 76.9 to 92.5% for palygorskite and from 71.2 to 90.2% for sepiolite, depending on the substrate concentration applied. The presence of Cd decreased the affinity to the substrate of both the free and the adsorbed ALP, while the maximum reaction velocity remained nearly unchanged, indicating that the inhibitory effects of Cd on both the free and adsorbed ALP activities were competitive in nature. The adsorbed enzyme, however, was inhibited less severely by Cd compared to the free enzyme. The adsorption of ALP on the fibrous clay minerals, therefore, maintains the ALP activity to a great extent and provides more resistance for the enzyme against the inhibitory effects of Cd.





Similar content being viewed by others
REFERENCES
Allison, S. D. (2006). Soil minerals and humic acids alter enzyme stability: implications for ecosystem processes. Biogeochemistry, 81, 361–373.
Amini, M., Afyuni, M., Khademi, H., Abbaspour, K. C., & Schulin, R. (2005). Mapping risk of cadmium and lead contamination to human health in soils of Central Iran. Science of the Total Environment, 347, 64–77.
An, N., Zhou, C. H., Zhuang, X. Y., Tong, D. S., & Yu, W. H. (2015). Immobilization of enzymes on clay minerals for biocatalysts and biosensors. Applied Clay Science, 114, 283–296.
Bouza, P., Simón, M., Aguilar, J., Del Valle, H., & Rostagno, M. (2007). Fibrous-clay mineral formation and soil evolution in Aridisols of northeastern Patagonia, Argentina. Geoderma, 139, 38–50.
Carrasco, M., Rad, J., & Gonzalez-Carcedo, S. (1995). Immobilization of alkaline phosphatase by sorption on Na-sepiolite. Bioresource Technology, 51, 175–181.
Cengiz, S., Çavaş, L., & Yurdakoç, K. (2012). Bentonite and sepiolite as supporting media: Immobilization of catalase. Applied Clay Science, 65–66, 114–120. https://doi.org/10.1016/j.clay.2012.06.004.
Chen, J., & Jin, Y. (2010). Sensitive phenol determination based on co-modifying tyrosinase and palygorskite on glassy carbon electrode. Microchimica Acta, 169, 249–254. https://doi.org/10.1007/s00604-010-0320-6.
Chipera, S. J., & Bish, D. L. (2001). Baseline studies of the clay minerals society source clays: powder X-ray diffraction analyses. Clays and Clay Minerals, 49, 398–409.
Coleman, J. E. (1992). Structure and mechanism of alkaline phosphatase. Annual Review of Biophysics and Biomolecular Structure, 21, 441–483.
Cornish-Bowden, A. (2013). Fundamentals of Enzyme Kinetics (4th ed.). John Wiley & Sons, Weinheim, Germany.
Datta, R., Anand, S., Moulick, A., Baraniya, D., Pathan, S. I., & Rejsek, K. (2017). How enzymes are adsorbed on soil solid phase and factors limiting its activity: A Review. International Agrophysics, 31, 287–302.
de Fuentes, I. E., Viseras, C. A., Ubiali, D., Terreni, M., & Alcántara, A. R. (2001). Different phyllosilicates as supports for lipase immobilisation. Journal of Molecular Catalysis B: Enzymatic, 11, 657–663.
Effron, D., De la Horra, A., Defrieri, R., Fontanive, V., & Palma, R. (2004). Effect of cadmium, copper, and lead on different enzyme activities in a native forest soil. Communications in Soil Science and Plant Analysis, 35, 1309–1321.
Farpoor, M., & Krouse, H. (2008). Stable isotope geochemistry of sulfur bearing minerals and clay mineralogy of some soils and sediments in Loot Desert, central Iran. Geoderma, 146, 283–290.
Ferrier, D.R. (2017). Lippincott's Illustrated Reviews: Biochemistry (7th ed.). Lippincott Williams and Wilkins, Philadelphia, USA.
Geiger, G., Livingston, M., Funk, F., & Schulin, R. (1998). β-glucosidase activity in the presence of copper and goethite. European Journal of Soil Science, 49, 17–23.
Gianfreda, L., & Bollag, J.-M. (1994). Effect of soils on the behavior of immobilized enzymes. Soil Science Society of America Journal, 58, 1672–1681.
Guggenheim, S. & Krekeler, M.P.S. (2011). The structures and microtextures of the palygorskite–sepiolite group minerals. Pp. 3–32 in: Developments in Clay Science Vol. 3 (E. Galàn and A. Singer, editors). Elsevier, Amsterdam.
Gülser, F., & Erdoğan, E. (2008). The effects of heavy metal pollution on enzyme activities and basal soil respiration of roadside soils. Environmental Monitoring and Assessment, 145, 127–133.
Huang, Q., & Shindo, H. (2000). Inhibition of free and immobilized acid phosphatase by zinc. Soil Science, 165, 793–802.
Huang, Q., Liang, W., & Cai, P. (2005). Adsorption, desorption and activities of acid phosphatase on various colloidal particles from an Ultisol. Colloids and Surfaces B: Biointerfaces, 45, 209–214.
Huang, Q., Zhu, J., Qiao, X., Cai, P., Rong, X., Liang, W., & Chen, W. (2009). Conformation, activity and proteolytic stability of acid phosphatase on clay minerals and soil colloids from an Alfisol. Colloids and Surfaces B: Biointerfaces, 74, 279–283. https://doi.org/10.1016/j.colsurfb.2009.07.031.
Karimi, A., Khademi, H., Kehl, M., & Jalalian, A. (2009). Distribution, lithology and provenance of peridesert loess deposits in northeastern Iran. Geoderma, 148, 241–250.
Kelleher, B. P., Willeford, K. O., Simpson, A. J., Simpson, M. J., Stout, R., Rafferty, A., & Kingery, W. L. (2004). Acid phosphatase interactions with organo-mineral complexes: influence on catalytic activity. Biogeochemistry, 71, 285–297. https://doi.org/10.1023/B:BIOG.0000049348.53070.6f.
Khan-Mohammadi, Z., & Nourbakhsh, F. (2011). Does salinity enhance Cd toxicity to soil alkaline phosphatase? Archives of Agronomy and Soil Science, 57, 753–762. https://doi.org/10.1080/03650340.2010.483592.
Khormali, F., & Abtahi, A. (2003). Origin and distribution of clay minerals in calcareous arid and semi-arid soils of Fars Province, southern Iran. Clay Minerals, 38, 511–527.
Kunze, G. & Dixon, J. (1986). Pretreatment for mineralogical analysis. Pp. 91–100 in: Methods of Soil Analysis: Part 1—Physical and Mineralogical Methods (A. Klute, editor). American Society of Agronomy. Madison, Wisconsin, USA.
Makboul, H., & Ottow, J. (1979). Alkaline phosphatase activity and Michaelis constant in the presence of different clay minerals. Soil Science, 128, 129–135.
McLaughlin, M.J. & Singh, B.R. (1999). Cadmium in soils and plants, a global perspective. Pp. 1–9 in: Cadmium in Soils and Plants (M.J. McLaughlin and B.R. Singh, editors). Kluwer Academic Publishers, Dordrecht, The Netherlands.
Naidja, A., Huang, P., & Bollag, J.-M. (2000). Enzyme-clay interactions and their impact on transformations of natural and anthropogenic organic compounds in soil. Journal of Environmental Quality, 29, 677–691.
Nannipieri, P. & Gianfreda, L. (1998). Kinetics of enzyme reactions in soil environments. Pp. 449–479 in: Structure and Surface Reactions of Soil Particles (P. Huang, N. Sensi, & J. Buffle, editors). John Wiley & Sons, Chichester, UK.
Nannipieri, P., Giagnoni, L., Landi, L., & Renella, G. (2011). Role of phosphatase enzymes in soil. Pp. 25–243 in: Phosphorus in Action: Biological Processes in Soil Phosphorus Cycling (E. Bünemann, A. Oberson, and E. Frossard, editors). Springer, Berlin Heidelberg.
Noble, J.E. & Bailey, M.J.A. (2009). Quantitation of protein. Pp. 73–95 in: Methods in Enzymology, Vol. 463 (R.R. Burgess and M.P. Deutscher, editors). Academic Press, San Diego, California, USA.
Nourbakhsh, F., & Monreal, C. M. (2004). Effects of soil properties and trace metals on urease activities of calcareous soils. Biology and Fertility of Soils, 40, 359–362. https://doi.org/10.1007/s00374-004-0786-7.
Pan, C., Liu, H.-D., Gong, Z., Yu, X., Hou, X.-B., Xie, D.-D., et al. (2013). Cadmium is a potent inhibitor of PPM phosphatases and targets the M1 binding site. Scientific Reports, 3, 2333. https://doi.org/10.1038/srep02333.
Rao, M. A., Gianfreda, L., Palmiero, F., & Violante, A. (1996). Interactions of acid phosphatase with clays, organic molecules and organo-mineral complexes1. Soil Science, 161, 751–760.
Ravankhah, N., Mirzaei, R., & Masoum, S. (2016). Spatial eco-risk assessment of heavy metals in the surface soils of industrial city of Aran-o-Bidgol, Iran. Bulletin of Environmental Contamination and Toxicology, 96, 516–523. https://doi.org/10.1007/s00128-016-1761-3.
Rosas, A., López, A., & López, R. (2010). Behavior of enzymatic activity in Chilean volcanic soil and their interactions with clay fraction. Pp. 313–328 in: Soil Enzymology (G. Shukla and A. Varma, editors). Springer. Berlin, Heidelberg.
Ruiz-Hitzky, E., Aranda, P., Álvarez, A., Santarén, J., & Esteban-Cubillo, A. (2011). Advanced materials and new applications of sepiolite and palygorskite. Pp. 393–452 in: Developments in Clay Science Vol. 3 (E. Galàn and A. Singer, editors). Elsevier, Amsterdam.
Sardar, K., Qing, C., Hesham, A. E.-L., Yue, X., & He, J.-Z. (2007). Soil enzymatic activities and microbial community structure with different application rates of Cd and Pb. Journal of Environmental Sciences, 19, 834–840.
Schimel, J., Becerra, C. A., & Blankinship, J. (2017). Estimating decay dynamics for enzyme activities in soils from different ecosystems. Soil Biology and Biochemistry, 114, 5–11.
Secundo, F. (2013). Conformational changes of enzymes upon immobilisation. Chemical Society Reviews, 42, 6250–6261.
Sedaghat, M., Ghiaci, M., Aghaei, H., & Soleimanian-Zad, S. (2009). Enzyme immobilization. Part 4. Immobilization of alkaline phosphatase on Na-sepiolite and modified sepiolite. Applied Clay Science, 46, 131–135.
Shirvani, M., Shariatmadari, H., Kalbasi, M., Nourbakhsh, F., & Najafi, B. (2006). Sorption of cadmium on palygorskite, sepiolite and calcite: equilibria and organic ligand affected kinetics. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 287, 182–190.
Smolders, E. & Mertens, J. (2013). Cadmium. Pp. 283–312 in: Heavy Metals in Soils: Trace Metals and Metalloids in Soils and their Bioavailability (B.J. Alloway, editor). Springer, Dordrecht, The Netherlands.
Stevenson, F. J., & Cole, M. A. (1999). Cycles of Soils: Carbon. Nitrogen, Phosphorus, Sulfur, Micronutrients: John Wiley & Sons.
Suárez, M., & García-Romero, E. (2012). Variability of the surface properties of sepiolite. Applied Clay Science, 67, 72–82.
Sumner, M. & Miller, W. (1996). Cation exchange capacity and exchange coefficients. Pp. 1201–1229 in: Methods of Soil Analysis. Part 3 (D.L. Sparks, editor). SSSA and ASA, Madison, Wisconsin, USA.
Tan, X., He, Y., Wang, Z., Li, C., Kong, L., Tian, H., Shen,W., Megharaj, M. & He, W. (2018). Soil mineral alters the effect of Cd on the alkaline phosphatase activity. Ecotoxicology and Environmental Safety, 161, 78–84, https://doi.org/10.1016/j.ecoenv.2018.05.069.
Tietjen, T., & Wetzel, R. G. (2003). Extracellular enzyme-clay mineral complexes: enzyme adsorption, alteration of enzyme activity, and protection from photodegradation. Aquatic Ecology, 37, 331–339.
Vandana, L., Rao, P., & Padmaja, G. (2012). Effect of herbicides and nutrient management on soil enzyme activity. New Facets of 21st Century. Plant Breeding, 5, 51.
Wang, Z., Li, Y., Tan, X., He, W., Xie, W., Megharaj, M., & Wei, G. H. (2017). Effect of arsenate contamination on free, immobilized and soil alkaline phosphatases: activity, kinetics and thermodynamics. European Journal of Soil Science, 68, 126–135.
Xin, Z., Liu, J., Shi, X., Wang, W., & Li, Y. (2016). Effects of Biochar on Soil Enzyme Activity and Mechanism of Action. Agricultural Science & Technology, 17, 2085.
Xin, J., Zhao, X., Tan, Q., Sun, X., Wen, X., Qin, S., & Hu, C. (2017). The effects of cadmium exposure on cadmium fractionation and enzyme activities in the rhizosphere of two radish cultivars (Raphanus sativus L.). Bulletin of Environmental Contamination and Toxicology, 98, 290–295.
Xu, J., Li, W., Yin, Q., Zhong, H., Zhu, Y., & Jin, L. (2007). Direct electron transfer and bioelectrocatalysis of hemoglobin on nano-structural attapulgite clay-modified glassy carbon electrode. Journal of Colloid and Interface Science, 315, 170–176.
Yalçin, H. & Bozkaya, Ö. (2011). Sepiolite–palygorskite occurrences in Turkey. Pp. 175–200 in: Developments in Clay Science Vol. 3 (E. Galàn and A. Singer, editors). Elsevier, Amsterdam.
Yu, W. H., Li, N., Tong, D. S., Zhou, C. H., Lin, C. X. C., & Xu, C. Y. (2013). Adsorption of proteins and nucleic acids on clay minerals and their interactions: A review. Applied Clay Science, 80, 443–452.
Zheng, L., Li, Y., Shang, W., Dong, X., Tang, Q., & Cheng, H. (2019). The inhibitory effect of cadmium and/or mercury on soil enzyme activity, basal respiration, and microbial community structure in coal mine–affected agricultural soil. Annals of Microbiology, 69, 849–859.
Zimmerman, A.R. & Ahn, M.-Y. (2011). Organo-mineral–enzyme interaction and soil enzyme activity. Pp. 271–292 in: Soil Enzymology (G. Shukla, & A. Varma, editors). Springer. Berlin Heidelberg.
Funding
This study was funded by Isfahan University of Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
(Received 14 August 2019; revised 20 February 2020; AE: R. Kukkadapu)
Rights and permissions
About this article
Cite this article
Shirvani, M., Khalili, B., Kalbasi, M. et al. ADSORPTION OF ALKALINE PHOSPHATES ON PALYGORSKITE AND SEPIOLITE: A TRADEOFF BETWEEN ENZYME PROTECTION AND INHIBITION. Clays Clay Miner. 68, 287–295 (2020). https://doi.org/10.1007/s42860-020-00066-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42860-020-00066-w